Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Zootecnia Tropical
versión impresa ISSN 0798-7269
Zootecnia Trop. vol.32 no.2 Maracay jun. 2014
Integrated Pest Management to control Varroa destructor and its implications to Apis mellifera colonies
Manejo Integrado de Plagas para el control de Varroa destructor y sus implicaciones para las colonias de Apis mellifera
Sergio R. Ruffinengo1, Matías D. Maggi2,3*, Jorge A. Marcangeli 2, Martín J. Eguaras2,3, Judith Principal4, Carlos Barrios4, Fiorella De Piano5, Mitton, Giullia6
1 Universidad Nacional de Mar del Plata. Facultad de Ciencias Agrarias. Cátedra de Apicultura. Balcarce, Argentina.
2 Universidad Nacional de Mar del Plata. Facultad de Ciencias Exactas y Naturales. Laboratorio de Artrópodos. Mar del Plata, Argentina.
3 CONICET. Consejo Nacional de Investigaciones Científicas y Técnicas. Buenos Aires, Argentina.
4 Universidad Centroccidental Lisandro Alvarado (UCLA). Decanato de Ciencias Veterinarias. Estación de Apicultura. Lara, Venezuela.
5 Universidad Nacional de Mar del Plata. Facultad de Ciencias Agrarias. Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC).
6 Universidad Nacional de Mar del Plata. Facultad de Ciencias Agrarias. Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC). email: biomaggi@gmail.com
ABSTRACT
Integrated Pest Management (IPM) is a pest management system that, in the socioeconomic context of farming systems, the associated environment and the population dynamics of the pest species, utilizes all suitable techniques in a compatible manner as possible to maintain the pest population levels below those causing economic injury. This article covers the principal aspects of the interaction between Apis mellifera and Varroa destructor and it describes the classical control forms applied to reduce the mite negative impact on colonies. Some examples of IPM activities that have been done to control this parasite in the southeast of Buenos Aires province, Argentina have shown good results. Several products worldwide have shown good effectiveness as well. Nevertheless, there are certain risks and hazards inherent to their use, such as: their negative impact on human health, resistance phenomena, loss of beneficial insects and native fauna, environmental pollution and drug residues in the hive products harmful for human consumption. The development of acaricide resistance in V. destructor populations and the possibility of incorporating contaminants in colonies by means of this type of treatment have promoted the addition of new molecules to minimize these disadvantages. The application of organic acids, essential oils and their components have become a worthwhile alternative. It can be concluded that to achieve an integrated management of V. destructor entails a change of mind for beekeepers and the active participation of all actors involved in the beekeeping sector to promote scientific activities aimed to discovering and developing new tools to be incorporated in an IPM Program against V. destructor.
Key words: Apis mellifera colonies, Varroa destructor, Integrated Pest Management, synthetic acaricides.
RESUMEN
El Manejo Integrado de Plagas (MIP) es un sistema de manejo que en el contexto socioeconómico de los sistemas agrícolas, asociado al ambiente y a la dinámica poblacional de las especies plaga, utiliza las técnicas apropiadas de una manera compatible para mantener, las poblaciones de la plaga por debajo de los niveles que causan daño económico. Este artículo cubre los principales aspectos de la interacción entre Apis mellifera y Varroa destructor y describe las formas clásicas de control aplicadas para reducir el impacto del ácaro en las colonias. Algunos ejemplos de las actividades de manejo integrado de plagas realizadas para controlar este parásito en el Sureste de la provincia de Buenos Aires, Argentina, mostraron buenos resultados. Existen algunos productos acaricidas de síntesis en el mundo, que presentaron buena efectividad. Sin embargo, el desarrollo de resistencia a los acaricidas en poblaciones de V. destructor y la posibilidad de incorporación de contaminantes en las colonias por este tipo de tratamientos, se han transformado en cuestiones de gravedad. Esto ha promovido la búsqueda de nuevas moléculas para minimizar estas desventajas. Ácidos orgánicos, aceites esenciales y sus componentes se han convertido en una valiosa alternativa. El éxito de la implementación de herramientas para el Manejo Integrado de Varroa involucra un cambio de mentalidad en los apicultores y la participación activa de todos los actores del sector apícola, para promover actividades científicas que ayuden a desarrollar nuevas alternativas para ser incorporadas en Programas Regionales de Manejo Integrado de esta parasitosis.
Palabras clave: Colonias de Apis mellifera, Varroa destructor, manejo integrado de plaga, acaricidas sintéticos.
Recibido: 23/10/14 Aprobado: 01/12/14
INTRODUCTION
The sexual reproduction of many crops and most wild plants depends on animal pollination by insects, birds, and bats, among others. Insects play the most important role in this respect (Klein et al., 2007). Among them, solitary and social bees provide the greatest contribution to the development of angiosperms (Brown and Paxton, 2009). This is explained in part, by the massiveness and homogeneity of modern agriculture, due to which most crops depend on honeybee-mediated pollination (Aizen et al., 2008). Even though global trends seem to indicate that bee population is growing (Aizen et al., 2009), there is strong evidence that a marked decline in pollinator populations has taken place in different parts of the world (Biesmeijer et al., 2006).
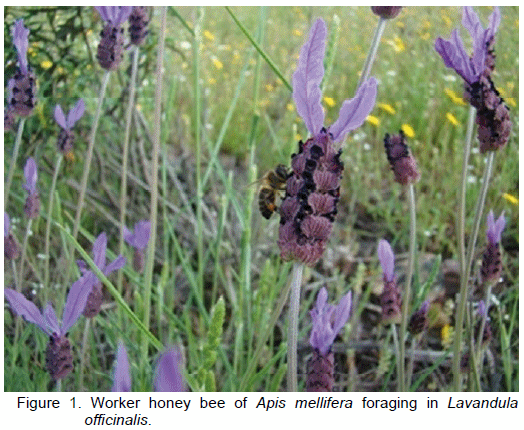
The European honeybee Apis mellifera L. (Figure 1) is the most important crop pollinator, with an exhaustively studied biology. Its distribution is wide, spanning from Europe all the way to Africa and Asia (Smith, 1991). Currently it can also be found in America, owing to colonies transfer by beekeepers for production purposes (Delaplane and Mayer, 2000). The relevance of A. mellifera for humanity lies in its being responsible for pollinating 77% of the food resources that sustain human population worldwide (Buchmann and Nabhan, 1996). However, as social individuals, colonies exert a double attraction for the pests and pathogens affecting them. On the one hand, colonies represent a high density of potential hosts, and, on the other, a large assembly of individuals with similar genetic characteristics (Schmid-Hempel, 1995). In the colony, resources abound, there being protein (pollen) and carbohydrates (honey), which are potential sources of food for other individuals circulating in the apiaries (Delaplane and Mayer, 2000).
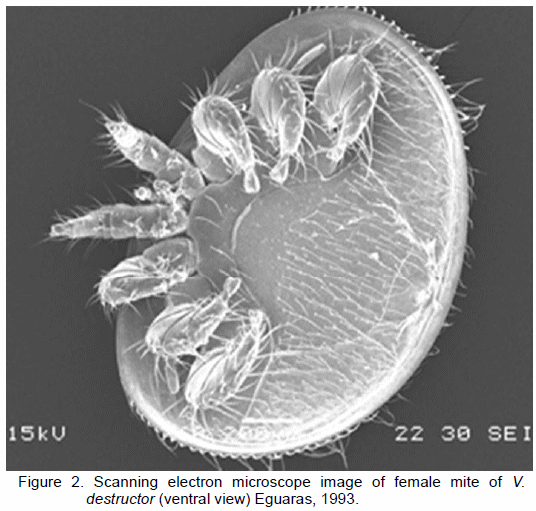
A. mellifera is affected by various living organisms. Among the most virulent ones, virus, bacteria, fungi, beetles and mites (Genersch, 2010) should be underlined. Mites parasitizing A. mellifera have become a severe concern worldwide, as they threaten the survival of bee colonies and jeopardize commercial beekeeping development (Sammataro et al., 2000). In view of the negative impact that Acarapis woodi (Rennie, 1921), Tropilaelaps clareae (Delfino and Baker, 1961), Varroa jacobsoni (Oudemans, 1904) and Varroa destructor (Anderson and Trueman, 2000) exert on bee colonies, these mites species have attracted the attention of the scientific community. Among the above mentioned, V. destructor (Figure 2) causes the most devastating effects on European bee colonies worldwide (Rosenkranz et al., 2010). The ectoparasitic mite V. destructor is native from the Asiatic bee Apis cerana, and was able to parasitize A. mellifera when beekeepers transferred European bees from Europe to the east of Russia in the first half of the last century, resulting in the sympatric distribution of both bees (Oldroyd, 1999). The negative effects of V. destructor on bees result from the lack of defensive co-evolutionary mechanisms that A. mellifera has, which is explained by the limited time during which they have been in contact (Rath, 1999). The mites life cycle can be divided into two stages: the first one is called the phoretic phase, where fertilized female mites are on adult bees and are able to spread among bees and / or colonies. The second one, so-called the reproductive stage, is that in which female mites enter brood bee cells to reproduce (Maggi et al., 2010a). Male mites have their quelicera adapted to transfer sperm, and use it to fecund female mites. Then, males die inside the cells due to starvation. The worldwide distribution of V. destructor is strongly connected to its own specialization and adaptation to phoresy (Akimov et al., 1988), but foremost to human intervention (Eguaras and Ruffi nengo, 2006). Broadly speaking, the negative effects generated by parasites vary and can be differentiated into: direct damage caused by the mite, and collateral damage resulting from mite infestation. The first category comprises weight loss, decreased halflife of parasitized bees (Marcangeli et al., 1992; Bowen-Walker and Gunn, 2001; Duay et al., 2002), and reduction of haemolymphatic proteins and haemocites with a subsequent lowering of the immune response in parasitized individuals (Gregory et al., 2005). The damage caused by mites on individual bees is mostly expressed in the larval and pupal stages. This depends on the number of mother mites parasitizing the brood. For instance, a single female V. destructor can be blamed for an average loss of 7% of bee weight (Rosenkranz et al., 2010). With regard to the indirect damages caused by the mite, V. destructor inoculates a wide variety of microorganisms by feeding processes. To date as many as 18 different viruses have been isolated in bee colonies (Chen and Siede, 2007), most of them vectored by Varroa. Prior to the appearance of mites in European colonies, viruses were not considered a health issue for A. mellifera (Yue and Genersch, 2005). Currently, it has been determined that different types of viruses live in bee colonies in latent form, and become highly infective when high mite infestation occurs in the beehive (Ball and Bailey, 1997). Additionally, V. destructor can induce bee immunosuppression, which would enhance virus infections (Yang and Cox-Foster, 2007). There is also clear evidence regarding V. destructor creation of ideal conditions for the development of the fungal pathogen Ascosphaera apis inside the beehive, the causative agent of chalkbrood breeding (Puerta et al., 1990). De Rycke et al. (2002) reported that mite is capable of carrying on it body Paenibacillus larvae spores, although it is not clear whether such load is sufficient to develop American foulbrood in healthy colonies. Recent studies have hypothesized that the hives critical loss seen in recent years in both Europe and USA would be the result of synergistic effects produced by the combination of V. destructor and the microsporidium N. ceranae (Anderson and East, 2008).
Since their introduction, synthetic plaguicides have been the preferred choice by human beings and pest control. Indeed, they play a key part in the full-scale agriculture production. Nevertheless, there exist certain risks and hazards inherent to their use, such as their negative impact on human health, loss of benefic insects and native fauna, environmental pollution, drug residues in products meant for human consumption and resistance phenomena.
The evolutionary time of the V. destructor/A. mellifera parasitic system is brief, and leads to the regulatory mechanism deficit of mite populations by their hosts (Peng et al., 1987). Therefore, to maintain healthy bee colonies, mite populations should be controlled by using acaricides. When parasitosis emerged in Europe and America, researchers postulated that a threeyear parasite cycle was necessary to cause the colony death (Ritter, 1981). Today it is known that unless colonies receive an acaricide treatment, they will die within the first year especially in temperate climates. Historically, the chemicals used by beekeepers to control Varroa mite were synthetic acaricides.These belong to different chemical types (pyrethroids, organophosphates, amidines, organic acids), and confer their own dynamics inside bee colonies. These features allow to elucidate the chemical behavior of each active ingredient inside the hive and to determine its residuality and likelihood of generating resistance phenomena in mite populations.
Initially, chemicals were supplied inside beehives by spraying, evaporation, dusting or spraying. With the passage of time, researchers have tried to attain better acaricide management methods inside colonies over time. By means of bees trophallaxis phenomenon (i.e., food exchange from bee to bee), acaricides were assayed on a systemic basis in the hive to ensure a rapid and even drug distribution. Nevertheless, one of the drawbacks that this administration form had, is that the acaricide is not able to kill mites inside the cells. Hence systemic acaricides are more suitable when used in the absence of breeding or when it is greatly reduced. Other control methods, based on the long stay of active ingredients in the hive, have allowed the active ingredient to act for a longer period of time, as mite emerges from brood cells. Several pyrethroids have shown a good acaricidal effect when incorporated into PVC strips between the frames of the brood chamber. Such administration is effective, even on beehives with breed throughout the year.
From late 1980´s to early 1990´s, the use of fluvalinate (a synthetic pyrethroid that acts on sodium channels) to control mite prevalence resulted in efficacies approaching 100% (Herbert et al., 1988). The high recorded efficacies, coupled with the easy application of these treatments inside bee colonies, led to an extensive use of this acaricide for years, generating a strong selective pressure on mite populations with the consequent appearance of mite-resistant phenotypes in many countries worldwide (Milani, 1999). Moreover, cross-resistance phenomena between pyrethroids fluvalinate and flumethrin in V. destructor populations were also reported (Milani, 1995, Thompson et al., 2002). In areas where these processes were identified, alternative methods, such as coumaphos (organophosphate acetylcholinesterase inhibitor) and amitraz (formimidina) were implemented (Elzen and Westervelt, 2002). However, the intensive and abusive use of these molecules to control Varroa mite, also led to resistance episodes in the United States, Mexico and Europe (Elzen et al., 1999; Mathieu and Faucon, 2000; Rodriguez-Dehaibes et al., 2005).
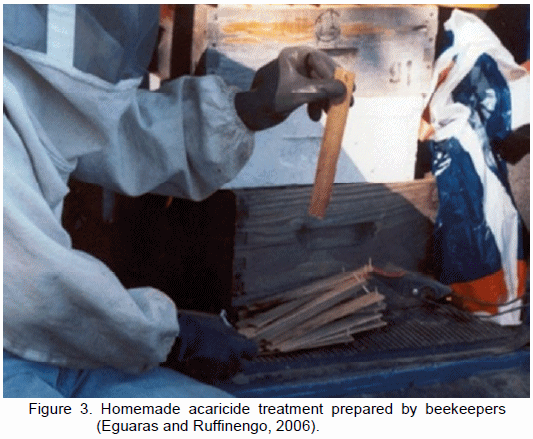
Another issue inherent to parasite control is the use of homemade drugs prepared by beekeepers (Figure 3). This practice is deeply rooted in beekeeping and is one of the most important causes explaining the development of the resistance phenomena of the mite populations being treated in Argentina (Eguaras and Ruffinengo, 2006). In addition to the resistance issues arising from the excessive use of synthetic acaricides, there exist residue problems in the different colonies matrices. Each acaricide treatment inevitably affects the quality of several hive products (Wallner, 1999). Beeswax is composed of a high fatty acid content of high molecular weight, which renders it suitable for lipophilic substances accumulation, such as coumaphos and fl uvalinate (Bogdanov, 2006). Moreover, honey is an aqueous matrix in which acaricides can solubilize hydrophilic chemicals (examples of these are organic acids and thymol). Thus, they can pass directly to the honey and affect the quality of the final product. These active ingredients have characteristics that make them much less harmful than stable lipophilic agents because their stability is significantly lower when compared to lipophilic acaricides (Eguaras and Ruffinengo, 2006).
Lipophilic acaricides are incorporated into the wax, are very stable and do not degrade easily in this medium. However, when using higher doses than those recommended, these agents can be accumulated. Wax has a large storage capacity of lipophilic substances, and wax recycling cans double the original amount of chemicals stored, depending on the chemical used (Imdorf et al., 2003). The most commonly detected acaricides in wax are fluvalinate and coumaphos (Wallner, 1999). The destruction of the honeycomb is probably the only way in which acaricides can be completely eliminated. Thus, alternative recycling forms should be sought, in order to, at least, reduce the concentration of the lipophilic toxic agents tested. A special mention should be made of amitraz. This lipophilic acaricide, belonging to the family of amidines, is very unstable in both wax and honey, and it degrades very quickly. The wax seems to have a significant accelerating effect on amitraz degradation (Wallner, 1999), since it is completely degraded in 1 day in this matrix, and, in honey, in about 10 days (Korta et al., 2001). Amitraz instability emerges as a key feature when honey analyses are made to detect this acaricide. Studies carried out by Maver and Poklukar (2003) reported very low levels or absence of amitraz residues in honey. Bee movement inside the hive also produces the distribution of chemicals which reach the different wax layers with which the comb surface is coated. This movement can cause propolis and virgen beeswax contamination (Wallner, 1999). Lipophilic substances can migrate from wax to honey, staying there for some time before they degrade, being detectable in amounts measurable by chemical analysis (Kochansky et al., 2001). Therefore, much emphasis should be placed on the stability that this chemicals have in a hydrophilic matrix like honey. Greek researchers found that fl uvalinate in honey remains stable for over 8 months remaining unchanged by the effect of temperature (Tsigouri et al., 2001). Korta et al. (2001) expanded this study to substances such as bromopropylate, coumaphos, chlordimeform and fl umethrin, and results for all of them displayed persistence in honey for about 9 months.
On the other hand, there is another type of lipophilic acaricides, those with volatile compounds like essential oils. During the application, their compounds evaporate very quickly and only a small amount remains in wax. The concentrations of these compounds can be detected in honey at very low rates. However, essential oils are substances with intense aromas and able to alter honey taste. Honey in which thymol, camphor and menthol had been added separately, showed a change in astringency and taste. Thymol was the one with the strongest effect on honey, producing a significant change in honey taste (Bogdanov et al., 1999). As a consequence, compounds such as thymol should be applied far away from nectar fl ow, to avoid major changes in taste.
The organic acids (formic, oxalic acid, lactic acid) often used to control V. destructor are also natural components of honey, and their concentration can vary within a wide range, depending on the type of honey. They are difficult to detect in amounts higher than those naturally occurring in honey, but just like essential oils, they generate changes in honey taste, especially when applied with formic acid near the honey flow. Properly used, thymol, formic and oxalic acids are excellent tools for the control of V. destructor (Eguaras and Ruffinengo, 2006).
The development of acaricide resistance in V. detructor populations and the possibility of incorporating contaminants in colonies by means of this type of treatment (Milani, 1999; Wallner, 1999) have promoted the addition of new molecules to minimize the disadvantages mentioned above. Among them, the application of organic acids (Boecking and Trainor, 2007a), essential oils and their components have become a worthwhile alternative (Imdorf et al., 1999; Eguaras and Ruffinengo, 2006). However, some studies have reported undesirable effects of organic substances when they were applied to A. mellifera colonies (Imdorf et al., 1999; Gregorc and Smodis-Skerl, 2007), which streeses the need to implement management strategies involving much more than a mere change of one molecule for another.
Integrated Pest Management (IPM) is a pest management system that, in the socioeconomic context of farming systems, the associated environment and the population dynamics of the pest species, utilizes all suitable techniques in as compatible a manner as possible and maintains the pest population levels below those causing economic injury (Dent 1991). In terms of strategies for pest control, IPM is the most modern concept. Its main objective is to apply the least amount of toxic substances, combined with the implementation of cultural practices, with a view to minimizing risks and hazards for human beings and the environment. IPM is being successfully applied in more than fifty countries, and is focused on prevention and non-chemical treatments. To achieve this goal, researchers include continuous controls and reports about environmental health, as well as pests recognition and their biology. Finally, with all this information, researchers are able to conduct a comprehensive analysis and implement the most appropriate and safe control strategy.
Even though IPM is not a basic biological pest control system using organic drugs, it is extremely important to include as many organic substances as possible in control tactics. Conceptually this entails a change of mind for beekeepers, and leads to the replacement of the improper use of synthetic pesticides with a complete program involving various substances and strategies to maintain parasite populations below the economic damage threshold. Currently, most beekeepers do not apply IPM to control Varroa, but stick to a scheduled treatment instead: the use of one or two pesticides, applied systematically in accordance with a rigid and predefined schedule, carrying out, in many cases, low parasite prevalence (percentage of parasite infestation) treatments and, hence, unnecessary.
According to Eguaras and Ruffinengo (2006), four main points remain the cornerstone for a successful IPM for V. destructor populations:
Tactics to reduce the growth of V. destructor populations (biotechnical methods)
Monitoring and control, if applicable
Sanitary treatments with toxicologically and environmentally friendly substances.
Search for hosts (bees) tolerant to parasites.
MATERIALS AND METHODS
Biotechnical methods
These methods involve a series of colony manipulations to remove mites from the colony and limit parasite population growth. They are based on knowledge about V. destructor biology (i.e., preference for drone cells) or methods developed by beekeepers (multiplication of hives). These methods are usually tied to beekeeping hobbyists, but still some techniques could be incorporated in an integrated pest management program at a certain time of the year. A method used to reduce mite populations is the usage and replacement of combs with large numbers of drone cells. It is primarily based on parasites predilection for this type of brood cells. It is estimated that for every mite entering a workers brood cell, about 7 or 8 mites enter a drone cell (Eguaras and Ruffinengo, 2006). This natural phenomenon is worth considering since by eliminating drone cells, Varroa population could be reduced in a natural manner.
Another method to reduce mite population, quite similar to the one described earlier, consists in incorporating drone combs and subsequently removing them from the colonies once they are capped. This methodology also takes advantage of parasites preference for drone cells, and works well to reduce the number of mites in the hives. However, just like the previous one, it does not seem to be sufficient to effectively control parasitic infections and should be complemented with another type of control. However, the continuous addition and removal of drone combs may decrease parasite populations. Calis et al. (1999) applied the technique of consecutively replacing 5 drone comb frames, and was able to reduce the parasite population by 93%. The greater the number of combs used, the more effective the method. This is widely used in Cuba, where beekeepers place one drone comb each month in the colony and keep the parasite population under control (Verde Jiménez and Bande Gonzalez, 2005). The use of combs traps, so-called entrapment method, is quite similar to the one just described only that the bee queen is excluded. By means of this procedure, beekeepers are able to manage the amount of brood available for V. destructor infestation. It consists in removing the queen for 8 to 9 days in a box containing the comb, thereby forcing it to lay its eggs in the comb. Then, after the cells have been capped and before the young bees emerge, the frame is removed from the hive.
This procedure can be repeated three times, and has yielded good results in some European countries. The three stages of this method have been sketched and described by Fries (1993) as follows: the queen is introduced, together with a clean comb (without brood), in a queen excluder (this box limits the movement of the bee queen only to the comb) for about 8 to 9 days, during which the brood is close to be capped. The second stage involves removing the comb from the excluder and placing it elsewhere in the beehive brood chamber, placing a clean comb inside the excluder (with the queen). The third stage takes place 8 to 9 days later. At this moment the first comb is removed from the hive (and, together with it, the parasites infesting the capped brood).
The second comb is now transported to the brood chamber and again, a third clean comb is introduced inside the excluder. Eight or nine days afterwards, the second comb is also removed from the hive and the third one is transferred to the brood chamber. At this time, the excluder is taken out from the beehive and the queen is released. Finally 8 days later, beekeepers need to remove the third comb trap from the beehive. This system has come to remove approximately 90% of mites in the hive (Fries and Hansen, 1989). Nevertheless, no more than three combs should be used, because bee population declines markedly in hives.
The annual queen bee replacement method is based on the reproductive biology of A. mellifera. In the first year, the bee queen only lays a limited number of male eggs (drones) and indirectly controls mite infestation increase. Moreover a greater reproductive activity of a young queen translates into a strong and, bigger colony less susceptible to disease (Eguaras and Ruffinengo, 2006). Cutting a brood comb section is a technique that stimulates bees to build new brood cells generally designed for the drone. Once the cells are capped, the section of the comb is cut and burned outside the hive, removing a portion of the mite population in it. This procedure can be repeated in a few pictures during drones breeding season (Eguaras and Ruffinengo, 2006).
Nuclei formation is another way to reduce pest populations in the original colonies. This technique is generally conducted during late spring and early summer when the colony is growing up. It has been noticed that, during certain times of the year, with the formation of a nucleus per hive using two capped brood, the original parasite population may be reduced by 30% (Eguaras and Ruffinengo, 2006).
Monitoring and control, if applicable
Regarding V. destructor field management, the best tool beekeepers have is monitoring parasite populations. This basic tool enables the early detection of parasites in the hive, before irreversible damage occurs, keeping mite population below the economic damage threshold (Delaplane et al., 2005). The three methods most often used are: (1) killing mites in the colony with chemical products; (2) estimating mite population by sampling immature (drone and worker brood cells) and adult bees; and (3) sampling mite natural death rate. Several detection methods may be used (Branco et al., 2006) with a greater or lesser degree of complexity. V. destructor population dynamics depends on whether conditions as well as on the type of bee race (Moretto et al., 1991). Hence each geographical area should be aware of its own economic damage threshold, as differences in climate and bee ecotype could exist, resulting in different Varroa populations growth.
Sanitary treatments with toxicologically and environmentally friendly substances
Natural compounds represent a valid alternative and a useful tool that can be incorporated into an integrated pest management program which contemplates the rotation of existing synthetic acaricides and minimizes their use. They have low toxicity to mammals, little environmental impact and good public acceptance (Isman, 2000). Many of these natural compounds showed effects on parasitic bee mites, especially organic acids and some essential oils (Eguaras et al., 2001a, Eguaras et al., 2005; Ruffinengo et al., 2005). Essential oils are volatile liquid fractions, usually distillable by steam distillation or hydrodistillation by water vapor, which contain the substances responsible for plant aroma. These substances can be found in fl owers, seeds, fruits, leaves, bark and roots (Imdorf et al., 1999). They have been long used as insect repellents. However, recent researches conducted in several countries have also confirmed that they have insecticidal, bactericidal and fungicidal activity (Isman, 2000). Toxicity in insects is based on a neurotoxic effect. Some monoterpenes constituents of essential oils are competitive inhibitors of the acetylcholinesterase of the nervous system.
A research carried out by Enan et al. (1998), points to the octopaminergic nervous system as insects action site. With respect to V. destructor, several studies were based on natural essences or its components. By 1998 over 150 essences had been evaluated in vitro or in vivo (Imdorf et al., 1999), a number that has steadily grown in recent years (Ruffinengo, 2010). Inside hives, oils have been applied by spraying, incorporated with food, and placed inside porous and gel matrices with varying results (Figure 4). However, very few have been successful in controlling mite populations (Imdorf et al., 1999). Despite the fact that essential oils yielded encouraging acaricide effects when they were tested in vitro, they showed high variability in their final efficacy when they were applied inside hives (Mutinelli et al., 1994). The essential oil composition of each plant species tends to be unique. Still some species have different chemotypes which are characterized by certain variations in their components (Imdorf et al., 1999).
The chemical composition of an essential oil depends on the state of development of the plant from which it is extracted, on the parts being used, on the plant cropping method as well as on the climatic conditions affecting it (Duran et al., 2007). Steam distillation, solvent extraction and pressure produce variations in oils composition. Undoubtedly the lack of a steady chemical composition explains the variations in the results obtained by different researchers (Imdorf et al., 1999), and so the lack of effective strategic plans for the control of Varroa parasitizing A. mellifera. However, certain essential oil components have revealed good efficacy in vivo and in vitro Even though the major components of an essential oil truly refl ect the biophysical and biological characteristics of the oil from which it has been isolated (Ipek et al., 2005), the extent of its impact remains dependent on its concentration when it is tested alone or within essential oils (Bakkali et al., 2008).
Thymol is a phenolic monoterpene present in many plants such as thyme (Thymus vulgaris), basil (Osimum basilicum), rosemary (Rosmarinus officinalis), peppermint (Mentha piperita), sage (Salvia officinalis). In the case of thyme, this compound can reach 50% of the total essential oil extracted from the plant and has a high insecticide, bactericide, fungicide and nematicide capacity. Thymol is the only component of essential oils widely used in beekeeping. Studies for V. destructor control have shown efficacies between 70% and 95% (Eguaras et al., 2004).
On the other hand, organic acids such as formic, lactic and oxalic acid are natural components of honey (Milani, 1999), and, therefore, nonpolluting substances for hive products. Another advantage is that there is little chance that V. destructor develops resistance to them (Milani, 1999). The acaricidal activity of organic acids has been tested both in vivo and in vitro experiments (Eguaras and Ruffinengo, 2006), and has yielded different efficacy results. This variability depends mainly on the acaricide concentrations applied, on the method of administration, bee ecotype and weather conditions (Rademacher and Harz, 2006). Fries and Hansen (1989) showed that the formic acid applied 4 times at a 24 hour interval is effective in controlling the parasite. Nevertheless, special care should be taken when formic and lactic acids are applied. Temperature drastically affects their release during treatment (Bahraini et al., 2004).
Oxalic acid has been successfully used in controlling V. destructor parasiting colonies of A. mellifera. However, both the concentration and dosage form of this molecule used in bee colonies play a key role in their effective miticide effect (Nanneti et al., 2003). Different studies have demonstrated that the best outcome in V. destructor population control is obtained when the acid is applied at concentrations below 4.5% m /v in sugar solution (De Feudis, 2007, Rademacher and Harz, 2006).
Search for hosts (bees) tolerant to pests
Another worth mentioning variable of the parasitic system evolution is bees defensive behavior (hygienic behavior). Hygienic behavior helps bees detect and remove mite from their hive. Mite recognition by bees is either for those placed on adult bees (grooming behavior) or inside brood cells (cleaning behavior) (Peng et al., 1987). Both mechanisms appear to combine and maintain low V.destructor population levels. Apparently the defensive behavior is more direct when it comes to killing parasites. Approximately 30% of the mites collected from the bottom of the hive featured different types of mutilations caused by bees. Hygienic behavior involves the ability of some bees to detect, uncap, and remove infested worker pupae from the brood cells. Apis cerana efficacy removes infested brood (Spivak, 1996). This removal behavior of infested pupae interrupts the reproduction of the fertile mites inside sealed brood cells. This rate in Africanized honeybees in Venezuela is about 60% (Principal et al., 2008). In addition, the immature mites are killed which decreases the average number of offspring per mother mite (Fries et al., 1994).
Another kind of mite tolerance by bees has also been reported by Ibrahim and Spivak, 2006 They mentioned in their work, that there is a line of bees (factor SMR) which maintains low mite levels because varroa appears to have low reproductive success on worker brood. They found such trait to be a heritable trait, and called it Suppression of Mite Reproduction (SMR). In colonies bred for SMR, mites entered worker brood cells to feed and reproduce. However, the authors reported that mites died in the cell without reproducing, produced no progeny, males only, or progeny too late to mature (Harris and Harbo, 1999; Harbo and Hoopingarner, 1997). The search for bees tolerant to Varroa is a continuous field of research whose aim is to find bee lines that help maintain mite populations at low levels.
RESULTS AND DISCUSION
Argentine beekeeping industry has boomed in recent decades, encouraged by a fall in the output of traditional producing countries and the subsequent price increase, which promoted the incorporation of several domestic producers (SAGPyA, 2009). World production of honey is about 1.4 million tons per year (FAO, 2008) and America is placed as the second honey producing continent. Data provided by the Cadena Apícola Santafesina (2008) has shown that Argentina contributes to honey production by 25%, accounting for 70% of the total production of South America. Beekeeping has placed Argentina among the top world producers, ranking first in the honey export segment (contributing to 20% of total exports) and third as a honey producers (contributing to 6 % of the world total production). Argentina exports 95% of its own honey production due to the low domestic consumption and the high demand coming mainly from European Union countries. In 2010, Argentina exported 57,487 tonnes of honey worth a total of 173 million dollars (SENASA, 2010).
V. destructor was first detected in Argentina in 1976. By that time, it was first found in hives located at Laguna Blanca, Formosa province (Montiel and Piola, 1976). It is believed that V. destructor had entered the country a few years before, considering that in 1971, beekeepers from Paraguay had imported honey bee queens from Japan introducing, for the first time, the mite in South America (Dietz, 1986). In Argentina, as in most countries, there are no colonies of bees able to stand the parasitic effects without severely damaging them. Therefore, mite populations should be controlled by beekeepers to avoid killing the colonies. The ultimate goal is to reduce these populations and bring them to acceptable levels that do not curtail productivity or bee colony survival.
In the framework of various research projects carried out in Argentina, the first investigations aimed at shedding some light on the biology of the parasite and its host. Studies on mite reproduction and mite seasonal variations helped the group better understand the population dynamics of parasites under different environmental conditions (Eguaras et al., 1996, Eguaras et al., 2001, Eguaras et al., 2003). Studies conducted by Eguaras (1988) reported mite behavioral adaptation to phoresy. Parasites preferred certain bee body parts to stay in phoresy. At a population level, it has been shown that the number of infertile females can vary on a seasonal basis along with egg-laying (Eguaras et al. 1994a, Eguaras et al., 1994b, Eguaras et al., 1995).
Maggi et al. (2009) also detected different morphotypes of Varroa mite populations in Argentina. The reason explaining these morphological variations among different populations remain unknown, but it has been hypothesized that the phenotypic expression may result from the genotypic interactions between the parasite DNA and its host DNA. Negative feedback mechanisms seem to be present at an infrapopopulation level. There is a strong crowding effect that leads to a reduction in the number of adult offspring per mother mite (Eguaras et al., 1995). Brood cells diameter has also been identified as a physical factor altering mite reproductive behavior (Maggi et al., 2010a). Taking into account all these experiences, the economic damage threshold for mite populations could be specified and strategies to mitigate the negative mite effects laid down (Eguaras and Ruffinengo, 2006).
Biotechnical methods, such as trap combs, have been applied with encouraging results in recent years. Using two combs trap, 65% of the total mites were removed from the colony. Greater effectiveness was attained in colonies with a higher number of parasites (Marcangeli, 2002). Adding drone combs trap, the effectiveness of this method increases. However, if worker combs trap are used, the method efficacy decreases (Damiani and Marcangeli, 2006). Moreover this method has the drawback of limiting the queen egg-laying activity to only drone brood for a certain period of time. Therefore, special care should be taken when using this methodology, given the resulting decrease in adult bees population that could occur in the colony. Hence, this method should only be used as part of a comprehensive program or supplemented with other control techniques.
Resistance phenomena to synthetic acaricides, such as coumaphos and amitraz, have been detected in Argentina and neighboring countries, such as Uruguay. This episode evidenced the need to seek new alternative molecules for parasite management (Maggi et al., 2008, Maggi et al., 2009, Maggi et al., 2010b, Maggi et al., 2011a). Different laboratory experiments have identifi ed promising essential oils (and/or its components) to be used against V. destructor (Eguaras et al., 2005, Ruffinengo et al., 2002). Ruffinengo et al. (2005) showed that essential oils obtained from Schinus molle and Acantholippia seriphioides had an interesting acaricidal effi cacy against V. destructor in vitro. These studies also reported that thymol and carvacrol were the main components of A. seriphioides essential oil, while
a- y β-phellandrene were major components of S. molle oil. Presently our group is evaluating the acaricidal effects of these isolated components and their toxic binary interactions against Varroa mite in vitro, as a strategy to solve the acaricidal variability efficacy reported for oils when they are applied in the fi eld (Eguaras and Ruffi nengo, 2006). New research lines are currently evaluating the application of native propolis and natural extracts as new antiparasitic agents against V. destructor in Argentina (Damiani, 2010a, Damiani et al., 2010b).Several field experiments have shown that thymol (Eguaras et al., 2004); oxalic acid (De Feudis, 2007) and formic acid (Eguaras et al., 2001, Eguaras et al., 2003) are an effective alternative tool for parasite population management. However special attention should be paid to external temperatures, concentrations of acaricides administered and colony population dynamics to avoid undesirable effects on bees. In the case of oxalic acid, the most important variable in the ultimate effectiveness of the formulation is the amount of brood present at the time of application (Marcangeli et al., 2003; Marcangeli and García 2004). When formic acid and thymol are used, high concentrations and high external temperatures can produce a large release of the acaricide in a short time and generate queen bee replacement, stop egg layment by queen and even bee colony swarming (Eguaras et al. 2001, Eguaras et al., 2003, Eguaras et al., 2004). Nowadays, Argentina counts with several synthetic acaricide products registered for controlling the mite V. destructor, to be applied in different ways on beehives. In spite of the diversity, the vast majority are based on three active agents: amitraz (Amivar®, Colmesan®, Varrotraz®, AB-Var®), coumaphos (Cumavar®, Perizin®), flumethrin (Flumevar®, Bayvarol®). Products with organic substances, based on oxalic acid(Oxavar®, Apioxal®) or Thymol (ABVar Bio®, Natural Var®), are rarely used by beekeepers.
Twenty years ago, registered commercial products were few and, besides, very expensive. This fact, led producers to manufacture controlling agents themselves (home-made products) in wood boards impregnated with flumethrine and fluvalinate or mixtures of cornflour with coumaphos, which were spread over the frames of the brood chamber. These procedures had many disadvantages: a) neither the dose nor the release of the drug were accurately controlled; b) many beekeepers bought the product ignoring their composition; c) the same drug was used year after year, without taking into account the product rotation; d) contaminant residues started to appear in honey and beewax; e) as years went by, the effi cacy of these treatments started to decrease.
As a result, animal health-care agencies (SENASA) and other official organizations (Ministerio de Asuntos Agrarios de la Provincia de Buenos Aires), as well as the Arthropods Laboratory of the Mar del Plata University, alerted the apiarian fi eld about the risks of this type of handling and the importance of an immediate change in Argentina. Within few years, several low-cost acaricides based on amitraz, coumaphos and flumethrine were registered. However, new problems emerged: some acaricides were effective in some areas while in some others their effi cacy was low or directly inexistent. When indiscriminately used along many years, the active agent loses effectiveness or causes the resistance of the mite towards the acaricide (Maggi et al., 2008). As a consequence, the Arthropods Laboratory began the implementation of acaricide effectiveness tests. Research took place mainly in two areas of Buenos Aires, one of them near La Plata city (northeast) and the other one near Mar del Plata city (southeast).
First tests were made with amitraz-made products. This drug, applied in continuous releasing strips, proved to have an efficacy under 90% (Mar del Plata: 85.05%; La Plata: 88.13%; Marcangeli et al., 2005). When used as a solution, the results were notoriously lower and with a huge variation depending on the beehive (La Plata: 70.02 ± 15.3; Marcangeli et al., 2005). A similar situation was the one presented by the use of fluvalinate (La Plata: 67.15%, Marcangeli and García, 2003).
Field trials with plastic strips with flumethrin showed better results and low variation between areas and colonies (Mar del Plata: 90.03% ± 2.3; La Plata: 88.25% ± 1.8; Damiani and Marcangeli, 2006). Still, there are registrations of low effectiveness associated with problems in the manufacturing of strips (Marcangeli, pers. ob.). Coumaphos also has provided good results in evaluated areas with values of 87.13% (La Plata) and 89.28% (Mar del Plata; Marcangeli et al., 2005). As a result, this drug was the most popular, what brought the first documented cases of resistance in Argentina (Maggi et al., 2008).
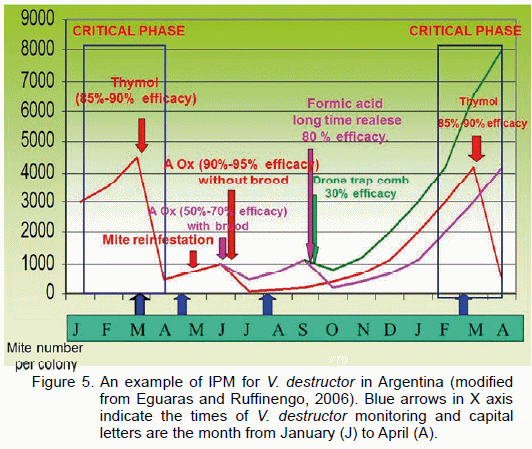
Nowadays, there are new cases of mite resistance towards agents of synthesis (Maggi et al., 2009, Maggi et al., 2010, Maggi et al., 2011b), resulting in a probable limited usage in time. Possible options were to be found in using natural substances such as essential oils, botanical extracts and propolis (Damiani, 2010a, Damiani et al., 2010b, Damiani et al., 2011). In preliminary tests, they proved to be good perspectives for their integration in a mite control system based on integrated pest management (Eguaras and Ruffinengo, 2006). Figure 1 depicts an example of an IPM carried out for V. destructor in the southeast of Buenos Aires province (Eguaras and Ruffinengo, 2006). In periods subsequent to honey collection, a standard colony can reach a mite population with close to 4000-5000 individuals. This represents the economic damage threshold for the colony. Indeed, this phase is critical for the colony and, therefore, a treatment should be administered to reduce the mite population to tolerable values. Currently daily counts of dead mites per colony are close to 20 mites/day. To avoid colony collapse, a treatment with thymol (1 application of 25 g of thymol in alcohol solution embedded in a spongy matrix) was conducted with an efficacy between 85% and 90%. As a result, the parasite population fell abruptly to around 450/600 mites per colony.
This treatment improves the colony condition and ensures the emergence of healthy bees to maintain a desirable colony population. However, getting through the winter and starting the spring in optimal conditions is not enough for the colony. The remaining number of mites in the hive, their continuous reproduction, coupled with the reinfestation caused by the proximity of wild swarms or neighbor apiaries, can alter the colony development. A second treatment with oxalic acid (4.5 % in sugar solution at 60%, 5 ml per comb) should be initiated during the cold weather when bee queens end egg-laying (usually June, early July). If during these months brood does not develop inside the colony, this single treatment with oxalic acid will suffice to reduce mite population (60/120 per colony) until the following year (March), and no subsequent treatment will be required in spring (see the red line in the figure). After oxalic treatment, mite population monitoring based on natural mite mortality is around 1 or 2 dead mites per hive. This value will increase to reach 20/25 mites per colony after harvest the following year. Conversely, if during winter breeding is significant, the oxalic treatment will have a reduced final efficacy (from 5o to 70 %), and the number of mites per colonies will only be reduced to 285/475 mites/colony. Indeed further monitoring should be implemented and possibly a new treatment with formic acid (purple line) or a biotechnical method (comb trap-green line) in early spring required.
CONCLUSION
Several fi eld experiments have shown that IPM developed for mite control can be used to maintain V. destructor populations below colonies damage levels. Nonetheless, more time should be spent and periodic visits be made to the apiary in order to implement the program. The IPM developed for beekeeping is a suitable tool even in areas where bee brood is present throughout the year. Taking this into account, a single treatment cannot successfully control parasites and, therefore, should be periodically repeated. Biotechnical methods and low bee toxicity products that do not add foreign elements to hive products should be adopted. Forms of control such as those developed in this chapter can assist in the reduction of the longstanding use of synthetic acaricides, reducing wax and honey residues as well as the resistance phenomena detected in V. destructor populations.
It has been demonstrated that the greater the effectiveness and success of arthropod pest management, the greater the likelihood of the pest developing resistance to that management tactics. This is particularly true when the goal of pest management is to reduce pest population and maintain it at a very low level. The probability of resistance evolution will be lower when goals emphasize damage and disease prevention, which sometimes can be accomplished without harming most of the pest population. In apiaries where Varroa mites are still susceptible, rotation between resistant and non-resistant acaricides (still effective in the control of the parasite) should prolong the effectiveness and prevent the occurrence of chemically resistant mites. In apiaries where Varroa mites are resistant, the introduction of Integrated Resistance Management (IRM) programs is essential. This includes selecting bees tolerant to the mite concerned, monitoring mite population, implementing nonchemical control methods and rotating pesticides, whether natural or synthesized.
Finally, achieving an integrated management of V. destructor entails a change of mind for beekeepers and the active participation of all those players involved in the industry. Producers should understand that the only way in which parasites can be managed is by implementing health strategies that address parasites and hosts biology, both of which are essential to attain an effective acaricide treatment. National and private scientifi c bodies should engage with the current issues faced by beekeeping and promote scientific activities aimed at discovering and developing new tools that could be implemented in an IPM. Finally, it is imperative that the political players responsible for national bee health ensure the linkage between the scientific and productive sectors so that the tools developed are implemented and honey bee preservation is ensured.
ACKNOWLEDGMENTS
The authors would like to thank the UNMDP and CONICET.
LITERATURE REVIEW
1. Aizen, M., L. Garibaldi, S. Cunningham and A. Klein. 2008. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Current Biology, 18 (20): 1572–1575. [ Links ]
2. Aizen, M., L. Garibaldi, S. Cunningham and A. Klein. 2009. How much does agriculture depend on pollinators? Lessons from longterm trends in crop production. Annasl of Botany, 103,(9): 1579–1588.
3. Akimov, I., A. Yastrebtsov and V. Gorgol. 1988. Functional and morphological specialization of Varroa jacobsoni for parasitism. In: Africanized honey bees and bee mites. Needham GR (Ed.), 474-478, Ellis Horwood, Chichester.
4. Anderson, D. and I. East. 2008. The latest buzz about colony collapse disorder. Science, 319 (5848): 724–725.
5. Anderson, D. and J. Trueman. 2000. Varroa jacobsoni is more than one species. Experimental and Applied Acarology, 24 (3):165–189.
6. Bahreini, R., J. Tahmasbi, M. Nowzari, and A. Talebi. 2004. A study of the efficacy of formic acid in controlling Varroa destructor and its correlation with temperature in Iran. Journal of Apicultural Research, 43 (4): 158-161.
7. Bakkali, F., S. Averbeck, D. Averbeck, and M. Ida omar. 2008. Biological effects of essential oils – A review. Food and Chemical Toxicology, 42(2): 446-475.
8. Ball, B. and L. Bailey. 1997. Viruses, In: Honey Bee Pests, Predators and Diseases. R.A. Morse and K. Flottum (Ed.), 11-32, Comstock Pub. Associates a division of Cornell University Press, Ohio, USA.
9. Biesmeijer, J., S. Roberts, M. Reemer, R. Ohlemueller, M. Edwards, T. Peeters, A. Schaffers, S. Potts, R. Kleukers, C. Thomas, J. Settele, and W. Kunin. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science, 313(5787): 351–354.
10. Boecking, O. and K. Trainor. 2007. Varroa biology and methods of control: part I of three parts. American Bee Journal, 147(10): 873-878.
11. Bogdanov, S. 2006. Contaminants of bee products. Apidologie, 37(1): 1–18.
12. Bogdanov, S., V. Kilchenman, P. Fluri, U. Bühhler, and P. Lavanchy. 1999. Infl uence of organic acid and essential oils on honey taste. American Bee Journal, 139(1): 61-63.
13. Bowen-Walker, P. and A. Gunn. 2001. The effect of the ectoparasitic mite, Varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomologia Experimentalis et Applicata, 101(3): 207- 217.
14. Branco, M., N. Kidd, and R. Pickard. 2006. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie 37 (4): 452-461. Brown, M. and R. Paxton. 2009. The conservation of bees: a global perspective. Apidologie, 40(3): 410–416.
15. Buchmann, S. and G. Nabhan. 1996. The Forgotten Pollinators. Island Press, ISBN 155-9633-53-0, Washington, DC. Cadena Apícola. Santafesina. 2008. Available on line: http://www.santafe.gov.ar/index.php/web/Estructura-deGobierno/Ministerios/Produccion/Informacion-General/PlanesEstrategicos-de-lasCadenas-de-Valor. Dic. 10, 2012.
16. Calis, J., I. Fries, and S. Ryrie. 1999. Population modelling of Varroa jacobsoni. Apidologie, 30(2-3): 111-124.
17. Chen, Y-P. and R. Siede. 2007. Honey bee viruses. In: Advances in Virus Research, Maramorosch. K.; Shatkin, A. and Murphy, F. (Ed.), pp. 33–80, Academic Press, Amsterdan, Netherland.
18. Damiani, N. and J. Marcangeli. 2006. Control del parásito Varroa destructor (Acari: Varroidae) en colmenas de la abeja Apis mellifera (Hymenoptera: Apidae) mediante la aplicación de la técnica de entrampado. Revista de la Sociedad Entomológica Argentina, 65(1-2): 33-42.
19. Damiani, N. 2010a. Control de Varroa destructor (Acari: Varroidae) mediante la utilización de propóleos. Tesis Doctoral. Universidad Nacional de Mar del Plata. 340 p.
20. Damiani, N., N. Fernández, L. Maldonado, A. Álvarez, M. Eguaras and J. Marcangeli. 2010b. Bioactivity of propolis from different geographical origins on Varroa destructor (Acari: Varroidae). Parasitology Research, 107(1): 31-37.
21. Damiani. N., L. Gende, M. Maggi, S. Palacios, J. Marcangeli and M. Eguaras. 2011. Repellent and acaricidal effects of botanical extracts on Varroa destructor. Parasitology Research.8(1): 79-86.
22. De Feudis, L. 2007. Control de Varroa destructor mediante la utilización de ácido oxálico. Tesis de Licenciatura en Ciencias Biológicas. Universidad Nacional de Mar del Plata. 50 p.
23. De Rycke, P., J. Joubert, S. Hossein Hosseinian and F. Jacobs. 2002. The possible role of Varroa destructor in the spreading of American foulbrood among apiaries. Experimental and Applied Acarology, 27(4): 313-318.
24. Delaplane, K. and D. Mayer. 2000. Crop polination by bees. CABI Publishing, Wallingford, U.K. Delaplane, K., J. Berry, J. Skinner, J. Parkman and W. Hood. 2005. Integrated pest management against Varroa destructor reduces colony mite levels and delays economic threshold. Journal of Apicultural Research, 44(4): 117-122.
25. Delfinado, M. and E. Baker. 1961. Tropilaelaps, a New Genus of Mite from the Philipppines (Laelaptidae (s. Lat.): Acarina). Fieldiana- Zoology 44(7): 53-56.
26. Dent, D. 1991. Integrated Pest Management. Chapman Hill, Andover, UK.
27. Dietz, A. 1986. The geographical distribution and levels of infestation of the mite Varroa jacobsoni Oudemans (Parasitiformes: Varroidae) in honey bee colonies in Argentina. American Bee Journal, 126(1): 49-51.
28. Duay, P., D. De Jong and W. Engels. 2002. Decreased flight performance and sperm production in drones of the honey bee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genetics Molecular Research 1(3): 227- 232.
29. Durán, D., L. Monsalve, J. Martínez and E. Stashenko. 2007. Estudio comparativo de la composición química de aceites esenciales de Lippia alba provenientes de diferentes regiones de Colombia, y efecto del tiempo de destilación sobre la composición del aceite. Scientia et Technica, 13(33): 435- 438.
30. Eguaras, M., and S. Ruffinengo. 2006. Estrategias para el control de Varroa. Editorial Martin. Mar del Plata, Argentina.
31. Eguaras, M. 1988. Varroasis en Apis mellifera. Tesis de grado. Licenciatura en Ciencias Biológicas. Universidad Nacional de Mar del Plata. 55 p.
32. Eguaras, M. 1993. Investigaciones sobre el ácaro parásito Varroa jacobsoni Oud. (Acari: Gamasida) y su hospedador Apis mellifera L. (Hymenoptera: Apidae). Tesis Doctoral. Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata. 345 p.
33. Eguaras, M., D. Cora, S. Ruffi nengo, C. Faverin and A. Palacio. 2004. Efectividad del timol en el control de Varroa destructor en condiciones de laboratorio y en colonias de Apis mellifera. Natura Neotropicalis 34-35: 27-32.
34. Eguaras, M., M. del Hoyo, A. Palacio, S. Ruffinengo and E. Bedascarrasbure. 2001. A New Product With Formic Acid For Varroa jacobsoni Control. I. Effi cacy. Journal of Medicine Veterinary,48(1): 11-14.
35. Eguaras, M. M. Labattaglia, C. Faverin, M. del Hoyo y E. Bedascarrasbure. 1998. Efectividad de los aplicadores Burmeister y Nassenheider en el control de Varroa jacobsoni. Revista Argentina de Producción Animal, 18:327-328.
36. Eguaras, M., J. Marcangeli and N. Fernandez. 1994. (a) Influence of the parasitic intensity on Varroa jacobsoni Oud. reproduction. Journal of Apicultural Research, 33(3): 155- 159.
37. Eguaras, M. J. Marcangeli, M. Oppedisano and N. Fernandez. 1994b. Seasonal changes in Varroa jacobsoni reproduction in temperate climates of Argentina. Bee Science, 3(2):120-123.
38. Eguaras, M., J. Marcangeli, M. Oppedisano and N. Fernandez. 1995. Mortality and reproduction of Varroa jacobsoni in resistant honeybee colonies (Apis mellifera) in Argentina. Bee Science, 3(2): 125-129.
39. Eguaras, M., M. Palacios, C. Faverin, M. Basualdo, M. Del Hoyo, G. Velis, and E. Bedascarrasbure. 2003. Effi cacy of formic acid in gel for Varroa control in Apis mellifera: importance of the dispenser position inside the hive. Veterinary Parasitology, 111( 2-3): 241-245.
40. Eguaras, M., S. Quiroga and García. 1996. The control of Varroa jacobsoni by means of organic acids. Apiacta 31(2): 51-54.
41. Eguaras, M., S. Ruffinengo, P. Bailac, G. Clemente, S. Fuselli, L. Gende, R. Fritz, A. Gonzalez and M. Ponzi. 2005. An in vitro evaluation of Tagetes minuta essential oil for the control of the honeybee pathogens Paenibacillus larvae and Ascosphaera apis, and the parasitic mite Varroa destructor. Journal of essential Oil research, 17(3): 336-340.
42. Elzen, P. and D. Westervelt. 2002. Detection of coumaphos resistance in Varroa destructor in Florida. American Bee Journal, 142(4): 291–292.
43. Elzen, P., J. Baxter, M. Spivak and W. Wilson. 1999. Amitraz resistance in Varroa: new discovery in North America. American Bee Journal, 139(2): 362.
44. Enan, E., M. Beiger and A. Kende. 1998. Insecticidal action of terpenes y phenols to cockroaches: effects of octopamine receptors. International Symposioum on Plant Protection, Gent, Belgium. FAO. Food and Agricultural organization. 2008. Available on line: http://www.fao.org/countryprofiles/index.asp?iso3=ARG. Dic. 10, 2012.
45. Fries, I. and H. Hansen. 1989. Use of trapping comb to decrease the population of Varroa jacobsoni in honey bee colonies in cold climate. Danish Journal of Plant and Soil Science, 93(2):193-198.
46. Fries, I. 1993. Nosema apis – a parasite in the honeybee colony. Bee World, 74(1): 5–19.
47. Fries, I., S. Camazine and J. Sneyd. 1994. Population dynamics of Varroa jacobsoni: a model and a review. Bee World, 75(2): 5-28.
48. Genersch, E. 2010. American Foulbrood in honeybees and its causative agent Paenibacillus larvae. Journal of Invertebrate Pathology, 103(1): 1-9.
49. Gregorc, A. and M. Smodis-Skerl. 2007. Toxicological and immunohistochemical testing of honeybees after oxalic acid and rotenone treatments. Apidologie, 38(3): 296–305.
50. Gregory, P., J. Evans, T. Rinderer and L. de Guzman. 2005. Conditional immune-gene suppression of honeybees parasitized by Varroa mites. Journal of Insect Science 5(7):1-5.
51. Harbo, J. and R. Hoopingarner. 1997. Honey bees (Hymenoptera: Apidae) in the United States that express resistance to Varroa jacobsoni (Mesostigmata: Varroidae). Journal of Economic Entomology, 90(4): 893-898.
52. Harris, J. and J. Harbo. 1999. Low sperm counts and reduced fecundity of mites in colonies of honey bees (Hymenoptera: Apidae) resistant to Varroa jacobsoni (Mesostigmata: Varroidae). Journal of Economic Entomology, 92(1): 83–90.
53. Herbert, E.W., W.A. Bruce and H. Shimanuki. 1988. Control of Varroa jacobsoni on honey bees in packages using Apistan®. American Bee Journal, 128(9): 615-616.
54. Ibrahim, A. and M. Spivak. 2006. The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie, 37(1): 31-40.
55. Imdorf, A., S. Bogdanov, R. Ibáñez Ochoa and N. Calderone. 1999. Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie, 30(2): 209- 228.
56. Imdorf, A., V. Kilchenman, R. Kuhn and S. Bogdanov. 2003. Beeswax replacement in organic bee keeping. Is there a risk of contamination by residues in hive walls?. Apiacta, 38(4): 178-182.
57. Ipek, M., A. Ipek, S. Almquist and P. Simon. 2005. Demonstration of linkage and development of the fi rst low-density genetic map of garlic, based on AFLP markers. Theorical and Applied Genetics, 110: 228-236.
58. Isman, M. 2000. Plant essential oils for pest and disease management. Crop Protection, 19: 603-608.
59. Klein, A., B. Vaissiere, J. Cane, I. Steffan-Dewenter, S. Cunningham, C. Kremen and T. Tscharntke. 2007. Importance of pollinators in changing landscapes for world crops. Procedings of the Royal Society B, Biological Science, 274(608): 303–313.
60. Kochansky, J., K. Wilzer and M. Feldlaufer. 2001. Comparison of the Transfer of Coumaphos from Beeswax into Syrup and Honey. Apidologie 32(2): 119–125.
61. Korta, E., A. Bakkali, L. Berrueta, B. Gallo, F. Vicente V. Kilchenmann and S. Bogdanov. 2001.Study of acaricide in honey. Characterization of amitraz degradation products in honey and beeswax. Journal of Agricultural and Food Chemistry, 49(12):5835-5842.
62. Maggi, M., N. Damiani, S. Ruffinngo, J. Principal, D. De Jong and M. Eguaras. 2010(a). Brood cell size of Apis mellifera modifi es the reproductive behavior of Varroa destructor. Experimental and Applied Acarology, 50(3): 269-279.
63. Maggi, M., L. Gende, K. Russo, R. Fritz and M. Eguaras. 2011a. Bioactivity of Rosmarinus officinalis essentials oils against Apis mellifera, Varroa destructor and Paenibacillus larvae related to the drying treatment of the plant material. Natural Product Research,25(4): 397-406.
64. Maggi, M., S. Ruffinengo, N. Damiani, N. Sardella and M. Eguaras. 2009. First detection of Varroa destructor resistance to coumaphos in Argentina. Experimental and Applied Acarology, 47(4): 317–320.
65. Maggi, M., S. Ruffinengo, L. Gende, M. Eguaras and N. Sardella. 2008. Baseline LC50 levels of Amitraz, Coumaphos, Fluvalinate and Flumethrine in populations of Varroa destructor from Buenos Aires Province, Argentina. Journal of Apicultural Research, 47(4): 292–295.
66. Maggi, M., S. Ruffinengo, P. Negri and M. Eguaras. 2010a. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitology Research 107 (5): 1189-1192.
67. Maggi, M., S. Ruffinengo, L. Gende, G. Sarlo, P. Bailac, M. Ponzi and M. Eguaras. 2010b. Laboratory evaluations of Syzygium aromaticum (L.) Merr. et Perry essential oil against Varroa destructor. Journal of Essential Oil Research, 22(2): 119–122.
68. Maggi, M., S. Ruffinengo, Y. Mendoza, P. Ojeda, G. Ramallo, I. Flores and M. Eguaras. 2011b. Susceptibility of Varroa destructor (Acari: Varroidae) to synthetic acaricides in Uruguay: Varroa mites potential to develop acaricide resistance. Parasitology Research, 108(4): 815-821.
69. Maggi, M., N. Sardella, S. Ruffi nengo and M. Eguaras. 2009. Morphotypes of Varroa destructor mites in different geographic locations from Argentina. Parasitology Research 105 (6): 1629. DOI: 10.1007/ s00436-009-1605-8.
70. Marcangeli, J. and M. García. 2003. Control del ácaro Varroa destructor (Mesostigmata: Varroidae) en colmenas de Apis mellifera (Hymenoptera: Apidae) mediante la aplicación de distintos principios activos. Revista de la Sociedad Entomológica Argentina, 62(3-4): 75-79.
71. Marcangeli, J. and M. García. 2004. Effect of Apis mellifera (Apidae) honeybee brood amount on Oxavar® acaricide efficacy against the mite Varroa destructor (Varroidae). :35-38.
72. Marcangeli, J. 2002. Evaluación de la técnica modificada de panales zanganeros para el control del ácaro Varroa destructor en colmenas de la abeja Apis mellifera. Revista de la Sociedad Entomológica Argentina, 61(1-2): 99-102.
73. J., M. García, G. Cano, L. Distefano, M. Martín, A. Quiroga, L. Raschia y C. Vega. 2003. Eficacia del Oxavar para el control del ácaro Varroa destructor en colmenas de Apis mellifera. Revista de la Sociedad Entomológica Argentina, 62(3-4): 75-79.
74. Marcangeli, J., M. García, C. Vega, A. Quiroga, M. Martín, L. Distefano and G. Cano. 2005. Estudio sobre la eficacia a campo del Amivar® contra Varroa destructor (Mesostigmata: Varroidae) en colmenas de Apis mellifera (Hymenoptera: Apidae). Revista de la Sociedad Entomológica Argentina, 64 (1-2):29-33.
75. Marcangeli, J., L. Monetti and N. Fernández. 1992. Malformations produced by Varroa jacobsoni on Apis mellifera in the province of Buenos Aires, Argentina. Apidologie, 23(5):399-402.
76. Mathieu, L. and J. Faucon. 2000. Changes in the response time for Varroa jacobsoni exposed to amitraz. Journal of Apicultural Research, 39(3-4): 155-158.
77. Maver, L. and J. Poklukar. 2003.Coumaphos and amitraz in Slovenian honey. Apiacta, 38(2): 54-57.
78. Milani, N. 1995. The resistence of Varroa jacobsoni Oud to pyrethroids: a laboratory assay. Apidologie 26(5): 415-429.
79. Milani, N .1999.The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 30(2): 229–234.
80. Montiel, J. and G. Piola. 1976. A new enemy of bees. Campo Moderno y Chacra, 1(1): 36- 37.
81. Moretto, G., L. GonÇalves and D. De Jong, 1991. The effects of climate and bee race on Varroa jacobsoni Oud. infestations in Brazil. Apidologie 22(3): 197–203.
82. Mutinelli, F., S. Cremasco and A. Irsara. 1994. Formic acid in the control of Varroatosis: a practical approach. Journal of Veterinary Medicine B, 41(7-8): 433-440.
83. Nanetti, A., R. Buchler, J. Charriere, I. Fries, S. Helland, A. Imdorf, S. Korpela and P. Kristiansen. 2003. Oxalic acid treatment for Varroa control. Apiacta 38: 81-87.
84. Oldroyd, B. 1999. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends in Ecology and Evolution, 14(8): 312–315.
85. Oudemans, A. 1904. On a new genus and species of parasitic acari. Note VIII. Leyden Mus 24: 216-222.
86. Peng, Y., Y. Fang, S. Xu, and L. Ge. 1987. The resistance mechanism of the Asian honey bee, Apis cerana Fabr. to an ectoparasitic mite, Varroa jacobsoni Oudemans. Journal of Invertebrate Pathology, 49(1): 54-60.
87. Puerta, F., J. Flores, J. Cuesta, M. Bustos y F. Padilla. 1990. Varroa, enfermedades secundarias. Vida apícola, 43:56-59.
88. Principal, J., C. Barrios, R. D´Aubeterre, S. Puzzar, S. B. García de la Rosa y S. R. Fuselli. 2008. Comportamiento higiénico de las abejas africanizadas (Apis mellífera scutellata Lepeletier) en apiarios del estado Lara, Venezuela. Zootecnia Tropical: 26 (2)167-173.
89. Rademacher, E. and M. Harz. 2006. Effectiveness of oxalic acid for controlling the Varroa mite. American Bee Journal, 146(7): 614-617.
90. Rath, W.1999 Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie, 30(2-3): 97-110.
91. Rennie, J. 1921.Isle of Wight disease in hive bees-acarine disease: the organism associated with the disease-Tarsoneus woodi, n.sp. Transactions of the Royal Society of Edinburgh, 52: 768-779.
92. Ritter, W. 1981. Varroa disease of the honeybee Apis mellifera. Bee World, 62(4): 141–153.
93. Rodríguez-Dehaibes, S., G. Otero-Colina, V. Sedas and J. Jiménez. 2005. Resistance to amitraz and flumethrin in Varroa destructor populations from Veracruz, México. Journal of Apicultural Research, 44: 124-125.
94. Rosenkranz, P., R. Aumeier and B. Ziegelmann. 2010. Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103(1): 96-119.
95. Ruffinengo, S. 2010. Evaluación de la bioactividad de aceites esenciales contra el ácaro Varroa jacobsoni. Tesis Doctoral. Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata. 348 p.
96. Ruffinengo, S., M. Eguaras, D. Cora, E. Rodríguez, E. Bedascarrasbure, P. Bailac and M. Ponzi. 2002. Biological activity of Heterotheca latifolia essential oil against Varroa jacobsoni. Journal of Essential Oil Research, 148(2): 462-464.
97. Ruffinengo, S., M. Eguaras, I. Flores, C. Faverin, P. Bailac and M. Ponzi. 2005. LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. Journal of Economic Entomology, 98(3): 651-655.
98. SAGPYA. 2009. Informe de la Dirección Nacional de Alimentos. Available on line: http://www.minagri.gob.ar/SAGPyA/economias_regionales/_apicultura/index.php. Dic. 10, 2012. Sammataro, D., U. Gerson and G. Needham. 2000. Parasitic mites of honey bees: Life history, implications, and impact. Annual Review of Entomology, 45:519-548.
99. Schmid-Hempel, P. 1995. Parasites and social insects. Apidologie, 26(3): 255-271.
100. SENASA. Servicio Nacional de Sanidad y Calidad Agroalimentaria. 2010. Estadísticas de Comercio exterior: exportaciones 2010. Available on line: http://www.senasa.gov.ar/estadistica.php. May. 05, 2011.
101. Smith, D. 1991. Diversity in the genus Apis. Westiew Press Inc, Boulder, CO. Spivak, M. 1996. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie, 27(4): 245–260.
102. Thompson, H., M. Brown, R. Ball and M. Bew. 2002. First report of Varroa destructor resistence to pyrethroids in the UK. Apidologie, 33(4): 357-366.
103. Tsigouri, A., U. Menkissoglu and A. Thrasyvoulou. 2001. Study of tau-fl uvalinate persistence in honey. Pest Management Science, 57(5): 467-471.
104. Verde Jiménez, M. y A. Bande Gonzalez. 2005. Lucha integrada de la Varroosis. I Congreso de Apicultura del MERCOSUR, Punta del Este, Uruguay, Junio 2005.
105. Wallner, K. 1999. Varroacides and their residues in bee products. Apidologie, 30 (2-3): 235- 248.
106. Yang, X. and D. Cox-Foster. 2007. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology,134(3): 405–412.
107. Yue, C. and E. Genersch. 2005. RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). Journal of General Virology, 86(12): 3419-3424.