Saber
versión On-line ISSN 2343-6468
Saber vol.28 no.1 Cumaná abr. 2016
LUIS FELIPE ESQUEDA1, SEBASTIAN LOTZKAT2, ANDREAS HERTZ2, MARCO NATERA3, JAVIER VALERA-LEAL4, ENRIQUE LA MARCA5, FERNANDO J.M. ROJAS-RUNJAIC6, RAMÓN RIVERO7
1 Investigador Independiente, Santiago de Chile, Chile, 2 Forschungsinstitut und Naturmuseum Senckenberg, Senckenberganlage, Frankfurt am Main, Deutschland, 3 Universidad Rómulo Gallegos, Museo de Vertebrados, San Juan de Los Morros, Venezuela, 4 Universidad Central de Venezuela, Facultad de Agronomía, Museo del Instituto de Zoología Agrícola - MIZA, Maracay, Venezuela, 5 Universidad de Los Andes, Facultad de Ciencias Forestales y Ambientales, Escuela de Geografía, Laboratorio de Biogeografía, Mérida, Venezuela, 6 Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Caracas, Venezuela, 7 Ministerio del Ambiente y de los Recursos Naturales Renovables, Museo de la Estación Biológica Rancho Grande, Maracay, Venezuela. E-mail: luisfesqueda@gmail.com
ABSTRACT
Pseudogonatodes lunulatus (Roux, 1927) was described from a single specimen collected in the village El Mene, Acosta municipality, northeastern Falcón state, Venezuela. Although the species has been reported for other Venezuelan and Colombian localities, an extensive study on taxonomy and geographic distribution in the country had not been performed. In this sense, we examined and compared a larger sample of 20 specimens deposited in several national and foreign museums. Additionally, we substantially expand the known distribution of the species to the north of the Orinoco River.
KEY WORDS: Pseudogonatodes, taxonomy, habitat.
VARIACIÓN MORFOLÓGICA Y DISTRIBUCIÓN GEOGRÁFICA DE Pseudogonatodes lunulatus (Roux, 1927) (SAURIA, SPHAERODACTYLIDAE) EN VENEZUELA
RESUMEN
Pseudogonatodes lunulatus (Roux, 1927) fue descrito de un único ejemplar recolectado en la población El Mene, municipio Acosta, al noreste del estado Falcón, Venezuela. Aunque la especie ha sido señalada para otras localidades de Venezuela y Colombia, un estudio extenso acerca de su taxonomía y distribución geográfica en el país no había sido efectuado. En este sentido, se pudo examinar y comparar una muestra mayor de 20 especímenes depositados en varios museos nacionales y extranjeros. Adicionalmente, se extiende sustancialmente la distribución conocida de la especie al norte del río Orinoco.
PALABRAS CLAVE: Pseudogonatodes, taxonomia, hábitat.
Recibido: julio 2015. Aprobado: octubre 2015 Versión final: febrero 2016
INTRODUCTION
At present, more than 370 species of reptiles are known from Venezuela (La Marca 1997, Kornacker 1999, Péfaur and Rivero 2000, Rivas et al. 2012, Natera-Mumaw et al. 2015). However, many of these taxa especially lizards were defined in base to succinct descriptions or poorly detailed, usually with few specimens examined and that did not reflect their current geographic distribution in the country (e.g. Anolis tropidogaster, vid. by Hallowell 1856; Polychrus marmoratus, vid. by Linnaeus 1758; Riama cephalolineata, vid. by García-Pérez y Yústiz 1995; Ameiva provitaae, vid. by García-Pérez 1995; Pseudogonatodes lunulatus, vid. by Roux 1927). Consequently, the taxonomic validity of some species has been questioned in time, as accur with Pseudogonatodes lunulatus (see Huey and Dixon 1970).
This last taxon was described on the basis of a single specimen collected by the Swiss geologist H.G. Kugler, as Lepidoblepharis lunulatus by Roux (1927), who already noticed its close relationship to Pseudogonatodes furvus Ruthven 1915 (species poorly described). Afterwards was considered a synonym of P. furvus by Parker (1935). Having examined five additional specimens of P. lunulatus collected by H. G. Kugler between 1939 and 1945, Shreve (1947) reconfirmed its distinctiveness regarding P. furvus, and its recognition within the genus. In subsequent decades, P. lunulatus was regarded as possible synonym of P. guianensis Parker 1935, by Test et al. (1966) and Huey and Dixon (1970), based on the apparent similarities between these taxa. Later, Dixon and Soini (1975) examined forty-one specimens corresponding to P. guianensis and whose data were compared with specimens of P. lunulatus. They believe in their study that both species are different, including a key for the genus. Is curious but none of these articles contains a formal description of P. lunulatus based on a number higher single specimen collected by the Swiss geologist H.G. Kugler, as Lepidoblepharis lunulatus by Roux (1927), who already noticed its close relationship to Pseudogonatodes furvus Ruthven 1915 (species poorly described). Afterwards was considered a synonym of P. furvus by Parker (1935). Having examined five additional specimens of P. lunulatus collected by H. G. Kugler between 1939 and 1945, Shreve (1947) reconfirmed its distinctiveness regarding P. furvus, and its recognition within the genus. In subsequent decades, P. lunulatus was regarded as possible synonym of P. guianensis Parker 1935, by Test et al. (1966) and Huey and Dixon (1970), based on the apparent similarities between these taxa. Later, Dixon and Soini (1975) examined forty-one specimens corresponding to P. guianensis and whose data were compared with specimens of P. lunulatus. They believe in their study that both species are different, including a key for the genus. Is curious but none of these articles contains a formal description of P. lunulatus based on a number higher of specimens of the previously known. Although Ávila-Pires (1995) did not make any judgment on P. lunulatus, only referred to the literature and accepted the taxonomic situation as presented at that moment. Regarding P. guianensis, she demonstrated the existence of geographic variation in several characters. For its part, Avila-Pires and Hoogmoed (2000) stated that they were unable to separate P. lunulatus from P. guianensis, therefore leaving them together in an identification key for the genus. Neither of these two papers tried to examine the validity of P. lunulatus. However, they discovered that a specimen now included in the type series of P. manessi had been mistakenly identified as P. lunulatus by Test et al. (1966), and consequently indicated by Manzanilla et al. (1996).
Regarding original description we present supplementary data on morphology, natural history and geographic distribution, based on the comparison of twenty nine specimens of different national and foreign museums.
MATERIALS AND METHODS
The descriptive structure follows previous studies of Huey and Dixon (1970), Ávila-Pires (1995), ÁvilaPires and Hoogmoed (2000) and Ávila-Pires (2001). We consulted Bustillos (1998) for the names of municipalities. Identification and analysis of vegetation was done following to Huber and Alarcón (1988) and Ataroff and Sarmiento (2003, 2004). The following abbreviations are used in the text: snoutvent length (SVL, from the tip of the snout to the anterior edge of the cloaca); head length (HL, anterior tip of rostral to the posterior edge of the ear- opening); head width (HW, distance between earopenings); forelimb length (FLL, from the axilla to the tip of the fourth finger), hind limb length (HLL, from the groin to the tip of the fourth toe), tail length (TL), horizontal diameter of the eye (HDE), eyenostril distance (END, measured from anterior edge of the eye to the nostril). All measurements were made using a caliper and rounded to the nearest 0.1 mm. Sex was determined by a direct examination of the gonads or with the help of a small cut on the abdomen.
The examined specimens are deposited in the following museum institutions: Colección de Anfibios y Reptiles del Laboratorio de Biogeografía (ULABG), Universidad de Los Andes, Mérida; Museo de la Estación Biológica de Rancho Grande (EBRG), Maracay, Aragua state; Museo del Instituto de Zoología Agrícola (MIZA), Universidad Central de Venezuela, Maracay, Aragua state; Museo de Historia Natural La Salle (MHNLS), Caracas, Dtto. Capital; American Museum of Natural History, New York, EE.UU. (AMNH); Field Museum Natural History, Chicago, EE.UU. (FMNH); Carnegie Museum of Natural History, EE.UU. (CM); Collection of Herpetology, University of California, The Angeles, EE.UU. (MVZ) and Collection of Herpetology, Kansas University, Museum of Natural History, EE.UU. (KU). The map drawn up to show the distribution of the species was made using the freely available program Diva-Gis v. 7.5.0. For its part, Venezuela's base map (shp format.) was conducted by the Venezuelan Institute for Scientific Research (IVIC), whose information is available to 2010 on the website http://www.ivic.gob.ve /ecology/lpydv/internal/?mod=galeriaMapas.php.
RESULTS
Pseudogonatodes lunulatus Roux, 1927 Verh. Naturf. Ges. Basel, 38: 252. (Figs. 1-5)
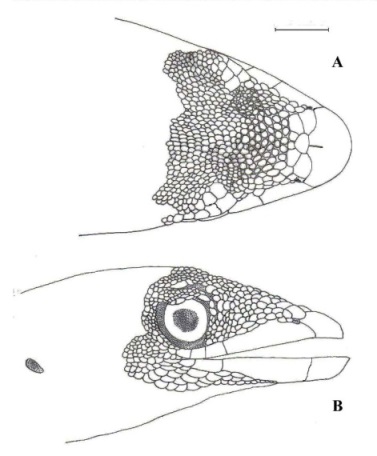
Figure 2. Pseudogonatodes lunulatus (Roux, 1927). A, B, C: ventral view of head, ventral surface of the right hand, and ungual sheath. CVULA 1775. Scale 1 mm.
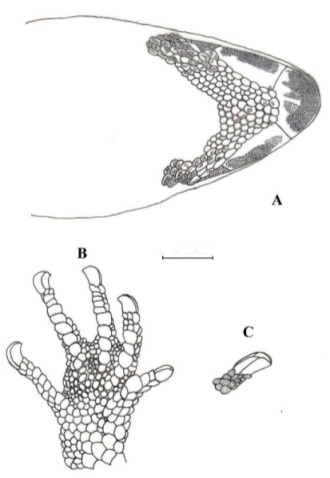
Figure 3. Pseudogonatodes lunulatus (Roux, 1927) coming from the Hacienda Guáquira in Yaracuy state: A: Field tag number SL 55; B, D: field tag number SL 85; C: field tag number SL 109 (s/n collection). Photographs Sebastian Lotzkat.

Figure 4. Ventral views of living Pseudogonatodes lunulatus (Roux, 1927) from the Hacienda Guáquira in Yaracuy state: A: Field tag number SL 85; B: field tag number SL 109 (s/n collection). Photographs Sebastian Lotzkat.
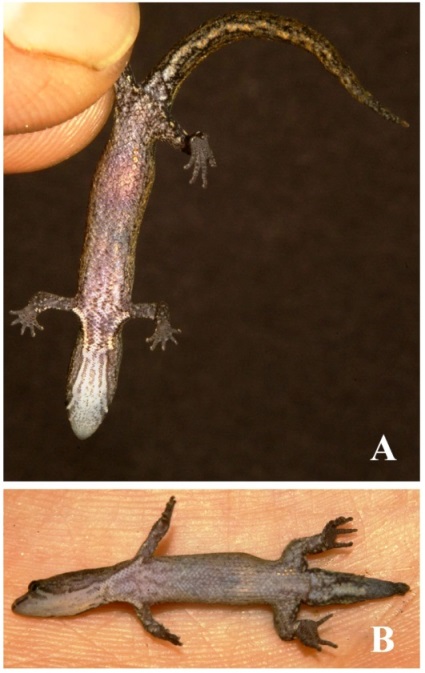
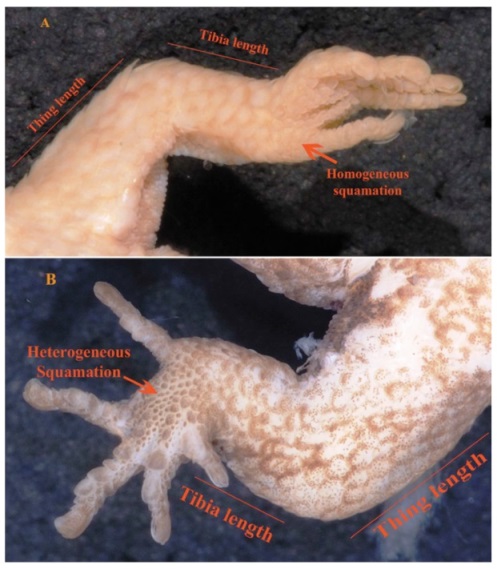
Figure 5. Hind limbs in its ventral view of Pseudogonatodes lunulatus, USNM 84970 (A) and Pseudogonatodes guianensis, USNM 538260 (B).
Material examined
ARAGUA state: Ocumare de la Costa de Oro municipality, Cata River, col. Fernando Rojas and Gilson Rivas, 11 October 2005, MHNLS 17481. MÉRIDA state: Obispo Ramo de Lora municipality, river basin Limones, 575 m asl, 0846'24"N and 7125'57"W, adult male, col. Diego Cadenas, Luis Felipe Esqueda and Enrique La Marca, 19 May 2004, ULABG 5738; 0846'24"N and 7125'57"W, adult male, col. Enrique La Marca, Enzo La Marca and Luis Felipe Esqueda, 15 April 2004, ULABG 6532; “Cacaotal” 1.7 km road La Azulita-Guayabures, to 12 km of La Azulita, 0846'48"N and 7125'94"W, adult female, col. Enrique La Marca, Diego Cadenas, Luis F. Esqueda and Enzo La Marca, 29 September 2004, ULABG 6600; sex not determined, col. Enrique La Marca, Mariella Márquez and Diego Cadenas, 23 October 2004, ULABG 6609; Andrés Bello municipality, “Selva Guayacán” (submontane forest), 0846'24"N y 7125'57" W, adult female, col. Enrique La Marca and Luis Felipe Esqueda, 05 February 2004, ULABG 6820; Antonio Pinto Salinas municipality, Santa Cruz de Mora, col. Erick Arrieta, 10 February 2003, MHNLS 16754. YARACUY state, Bolívar municipality, Aroa, Park Minas de Aroa, approx. 300 m asl, sex not determined, 25 July 1997, col. Ramón Rivero, EBRG 3845-3847; Granja El Renacer Mayorica, sex not determined, 03 July 2002, col. Ramón Rivero, EBRG 4196; CARABOBO state, Puerto Cabello municipality, Base Rancho Los Vaqueros, Borburata, 250 m asl, sex not determined, 18 September 1985, col. Ramón Rivero, EBRG 1923; VARGAS state, Los Canales, Naiguatá, 10°35'0"N and 66°40'0"W, CM 30118; MIRANDA state, 65 km SE de Caracas, P.N. Guatopo, MVZ 110732; SUCRE state, 0.8 km S de Cumaná, 34 m asl, 10°26'9"N and 64°9'55"W, FMNH 176865; Guaraunos, 29 m asl, 10°35'25"N and 63°7'23"W, KU 167478-9; MONAGAS state, Caripito, 50 m asl, 10°8'0"N and 63°6'0"W, AMNH 102592-94. REFERRED MATERIAL (not catalogued in museum): YARACUY state, Hacienda Guáquira 10 km east of San Felipe, near path along the Quebrada Ecológica, 120 m asl, 10°17'43"N and 68°39'11"W, adult male, col. Douglas Mora and Sebastian Lotzkat, 04 September 2006, field tag number SL 55; Hacienda Guáquira, 2 km along the same path, 380 m asl, 10°17'7"N and 68°38'29"W, sex not determined, col. Sebastian Lotzkat and Andreas Hertz, 06 October 2006, field tag number SL 85; Estación Ecológica Guáquira, 100 m asl, 10°17'51"N and 68°39'20"W, sex not determined, col. Sebastian Lotzkat and Andreas Hertz, 18 October 2006, field tag number SL 109 (see Lotzkat 2007).
Type locality
El Mene, Acosta municipality, Falcón state, Venezuela, by Roux (1927), Verh. Naturf. Ges. Basel, 38: 252, holotype NMBA 9338 (=NHMB). Additionally, municipality of other localities was corrected by Mijares-Urrutia and Arends (2000: 14).
Localities known in Venezuela
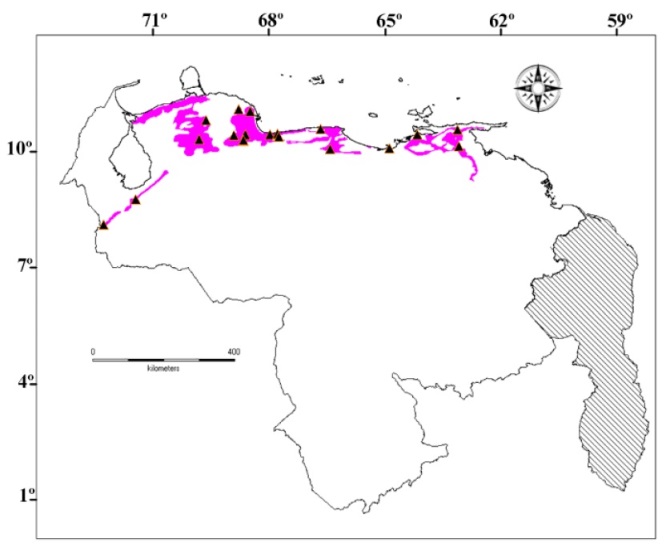
Occurs in the Cordillera de La Costa, Central stretch (Aragua, Carabobo, Miranda and Yaracuy states) and Oriental stretch (Anzoátegui, Sucre and Monagas states); as well as from the Serranía LaraFalcón and lacustrine versant of the Cordillera of Mérida (Táchira and Mérida states) (Fig. 6).
Figure 6. Geographical distribution of Pseudogonatodes lunulatus (Roux, 1927) in Venezuela. Black triangle material examined. Reddish area in accordance with the potential distribution of vegetation indicated by Huber & Alarcón (1988).
Etymology
The name probably derives from a pair of crescent-shaped pale marks on the back of the head. However, this character is not considered distinctive because it is present in other species of the group (e.g. P. furvus, P. guianensis).
Definition and diagnosis
This sphaerodactylid lizard may be differentiated by the following combination of characters: (1) third subdigital lamella under fourth toe expanded, (2) granular dorsal scales, not flattened; (3) 6-7 subdigital lamellae under fourth toe, (4) 3-4 postrostral scales, (5) sole of foot with homogeneous squamation; (6) tibia length foot moderate or longer, > 0.5 thigh length; (7) tongue distally notched, without fleshy protuberance; (8) usually three postmental scales, rarer four, (9) first supralabial scale posteriorly higher than length of the first infralabial; (10) eye-nostril distance 0.18-0.21 times HL; (11) first finger with the basal subdigital lamella dilated; (12) maximum SVL 21-29 mm; (13) 31-33 ventral scales between axilla and groin; (14) scales near snout bigger than those in interorbital and dorsal region; (15) expanded medial infracaudal scales, two times larger than lateral scales.
Pseudogonatodes lunulatus possesses an expanded third subdigital lamella and a total count of seven or less subdigital lamellae under the fourth toe, characters that immediately separate it from P. furvus Ruthven 1915, P. gasconi Avila-Pires and Hoogmoed 2000, P. manessi Ávila-Pires and Hoogmoed 2000 and P. peruvianus Huey and Dixon 1970 (cf. Ruthven 1915, Huey and Dixon 1970, Ávila-Pires and Hoogmoed 2000). It differs from P. barbouri because the latter possesses flattened dorsal scales (vs. conical to subconical in P. lunulatus).
Even though P. lunulatus and P. guianensis (between parenthesis) appear very similar, the first can be separated as it has sole of foot with homogeneous squamation, Fig 5A (vs. heterogeneous, Fig. 5B), tibia foot > 0.5 femur length, Fig. 5A (vs. < 0.5 regarding to the femur length, Fig. 5B) and first supralabial moderate or long, namely, > 0.5 to ≤ 0.7 regarding eye-nostril distance (vs. 0.5 or less).
Description
SVL: 21.4-29.9 (: 26.6; 3.78; n: 5), TL 0.831.03 (:0.86; 0.11; n: 5) times SVL, HL: 0.20-0.23 (: 0.21; 0.01, n: 5) times SVL, 1.25-1.55 (:1.43; 0.10; n: 5) times longer than wide. Forelimbs 0.17 0.21 (X: 0.20: 0.04; n: 5) times SVL and hind limbs 0.28-0.36 (: 0.30; 0.04, n: 5) times SVL.
Tongue relatively broad, with its anterior part extremely narrow, approximately 3/4 free, with a notch in the anterior edge, fleshy protuberance absent, dorsally covered by small imbricated papillae; teeth small, subequal and conical.
Rostral wider than high, visible from above, posterior edge with a short medial cleft (vertical to the scale); 3-4 postrostrals (Fig. 1A), the median one (s) smaller than the lateral ones; the latter (being equivalent to the supranasals) wider than long; adjacent scales to postrostrals smooth, flat, juxtaposed, bigger than scales in the interparietal and dorsal region; nasal small, nostril covers more than half of nasal surface, in contact with rostral, postrostral and first suparalabial; posteriorly (lateral view) in contact with two postnasals, these are bigger than adjacent loreal scales; eye-nostril distance 1.11.3 (X: 1.2; n: 5); snout with rounded scales, irregularly and flattened slightly, smooth, juxtaposed, subimbricate posteriorly; loreal region with flattened scales, smooth, juxtaposed, except those towards the eye, which are subconical; loreal scales arranged in 59 rows, in straight line from the posterior edge of postnasal to the orbit; 24-27 loreal scales; 11-17 scales between second supralabials; supraocular and interorbital region with subimbricate scales, mostly are high, being smaller with respect to scale toward the snout; upper border of eye formed by a group of supraciliaries scales, where one or two tend be clearly differentiated; 4/4 supralabials, posteriorly decreasing in size; anterior suture of the third and fourth supralabials under the eye; first supralabial moderate or long, namely, > 0.5 to ≤ 0.7 regarding eye-nostril distance (Fig. 1B); 3-5 suboculars, first and second enlarged; ear-opening small, twice taller than wide, directed obliquely forward; 13-14 scales between the edge of the labial commissure and anterior edge of ear opening.
Mental slightly wider than high, posterior edge irregularly straight or concave, 1-3 conspicuous clefts (absent in ULABG 6600); 3-4 postmental scales (Fig. 2A), well differentiated from the adjacent scales; 3-4 infralabials, first elongated, almost reaching anterior edge of the eye, slightly longer or longer first supralabial; throat region with scales rounded and juxtaposed; scales on neck and throat rounded, juxtaposed or subimbricate, smooth (similar to ventrals); a strong transition between both areas is clearly detectable.
Dorsal scales of body granular, some conical and erect, longer than the scales on posterior part of the head and temporal region; ventral scales subrhomboid, smooth, imbricate and arranged in oblique rows, bigger than dorsal scales; 66-90 middorsal scales; dorsolateral region (flanks) similar to dorsal surface; 31-33 ventral scales between forelimbs and hind limbs; 34-37 until cloaca; infracaudal scales of the midventral row bigger than lateral ones, flattened, smooth, imbricate and similar to ventral scales; supracaudal scales elongated, flat, smooth, differentiated from the dorsal scales, smaller than infracaudals; forelimbs extended forward almost reach or exceed the otic opening; tibia length moderate or long, almost equal to the thigh length (> 0.5 femur length, Fig 5A); forelimbs in their proximal third in dorsal view with smooth scales, imbricate, which are larger than the remaining ones; squamation towards the forearm tend to be conical; ventral surface of forelimbs with subconical scales much smaller than the ones previously mentioned; soles of hands and feet with homogeneous scales; subdigital lamellae of hands I3-3 II4-5 III7-7 IV7-7 V5-5, those of feet I3-3 II4-5 III6-8 IV6-7 V6-5; basal lamella of the first finger enlarged (Fig 2B); third lamella of the fourth toe expanded; claw enclosed by an ungual sheath composed of five scales.
Coloration in preservative (70% ethanol)
Dorsal region of head brownish from the neck to the snout, except by a pair of spots cream or yellowish in crescent-shaped; supralabials and infralabials barred, although there is an elongated cream line and obliquely arranged below the eye orbit; ventral surface of head creamy, except mental, first infralabial and postmentals which are strongly pigmented dark brown. Dorsal region of body uniformly light brown, but with two rows transversal cream marks; ventral surface of neck and belly cream, yellowish or light brown. Dorsal region of tail brown, slightly darker than the dorsum, but laterally with two cream lines; ventral surface of tail cream or yellowish, spotted dark brown; limbs brown on dorsum, ventrally light brown; palms and soles of hands and feet grayish brown or dark brown. Aterior third of tongue grayish, being cream the remaining portion.
Coloration in life
The following information is derived from several photographed specimens not yet catalogued (Figs. 34). The head dorsally is dark brown, being grayish brown between the eyes; two creamy or yellowish marks forming a crescent behind the eyes (dorsal view), which may be continuous or discontinuous, conspicuous or inconspicuous; usually barred pattern on the lip, but always with one pale line beginning at the posterior edge of the eye and obliquely extended in position latero-ventral, this may be well defined or barely visible. Dorsal surface of snout brownish sometimes sprinkled with white. Ventrally head grayish or whitish, but speckled of brown. In some adult individuals, the pale line on lip extends to the ventral region of the head where it fuses forming other semicircular line that reach forelimb (Fig. 4A). Iris reddish brown or dark brown with pupillary border yellowish brown or golden (black pupil). Tongue whitish with distally edge dark gray and forming a design in "U" (Fig. 3D).
Dorsal region of neck and trunk brownish, usually there are two rows of white marks, well defined or not and transversely disposed. Flanks brown, darker than dorsal region. Extremities dorsally brown speckled with white; ventrally pigmented of dark brown or grayish brown. Palms and soles of dark brown limbs.
Tail light brown to reddish brown (dorsal view), with two series of pale or yellowish cream blotches which are marginalized of dark brown (apparently are the continuation of the series of stripes found dorsolaterally on the trunk). Ventral side of the tail medially cream or reddish cream, irregular, sometimes interrupted toward the distal portion All Andean material was collected using pitfall traps (ULABG 5738, 6532, 6600, 6609, 6820), whose preserved fluid was made up mainly by ethanol and 25% formaldehyde, which allowed the specimens sustain their natural coloration, as follows: ventral surface of tail yellowish green, mottled dark brown. Throat, mental and infralabials yellowish green but these last peppered with dark brown. Spots on the head are cream yellowish, as well as the snout; vertical black bars are arranged on supralabials; soles of hands and foot grayish brown.
Habitat
The specimens from the Cordillera of Mérida were collected in two localities by using pitfall traps: a first area corresponding to submontane humid forest (following the classification of Ataroff and Sarmiento 2003, 2004) and a private area Finca El Palmar, 9.5 km from Santa Elena de Arenales”, whose original vegetation was intervened for the establishment of a plantation of cocoa (Theobroma sp.), near Limones River, Mérida state. Both places shared a high deposit of leaf litter. Additional reptiles and amphibians collected at these sites are Anolis aff. tropidogaster Hallowell 1856, Rhinella marina Linnaeus 1758, Engystomops pustulosus Cope 1864 and Leptodactylus sp.
For its part, specimens from Cerro Zapatero, Yaracuy state (Fig. 3 and Fig. 4) were collected from three different places at, and east of, the Estación Ecológica Guáquira (10°17'51"N, 68°39'20"W): The adult male SL 55 (Fig. 4A) was caught on 04 September 2006 in a pitfall trap in semideciduous lowland forest at 120 m asl, 10°17'42"N, 68°39'10"W. A few days later, the same pitfall trap contained the dead bodies of two diminutive lizards (without identified), which had been mauled beyond recognition by ants. While SL 85 (Fig. 4B, sex undetermined) was caught on 08 October 2006 in another pitfall trap in semideciduous premontane forest at 380 m asl. Both places are characterized by closed forest with a thick layer of leaf litter. Further herpetofauna encountered in the immediate surroundings of the pitfall traps at both spots comprises Allobates pittieri La Marca et al. 2004, Rhinella sternosignata Günther 1858, Phyllomedusa trinitatis Mertens 1926, Engystomops pustulosus Cope 1864, Leptodactylus cf. wagneri Peters 1862, Thecadactylus rapicauda Houttuyn 1782, Ptychoglossus kugleri Roux 1927, Anolis fuscoauratus D’Orbigny 1837, Anolis planiceps Troschel 1848, Plica plica Linnaeus 1758, Mastigodryas boddaerti Sentzen 1796, Ninia atrata Hallowell 1845, Sibon nebulatus Linnaeus 1758, and Bothrops colombiensis. Specimen SL 109 (Fig. 3C, sex undetermined) called for attention at noon on 18 October 2006, when it crossed the terrace of the Ecological Station itself, which is surrounded by cocoa plantations and grassland with sparse leaf litter, situated between the above mentioned forest and the swampy floodplains of Yaracuy River. From the station’s terrace, Engystomops pustulosus Cope 1864, Sphaerodactylus molei Boettger 1894, Thecadactylus rapicauda Houttuyn 1782, Ameiva ameiva Linnaeus 1758 and Cnemidophorus lemniscatus Linnaeus 1758 have been commonly observed (see Lotkzat 2007). A specimen (EBRG 3847) was extracted from the leaf litter by ants, which were carrying it towards the highway (field notes Ramón Rivero 1997).
During March 2006 a single individual (MIZA 0314) was observed active at daytime (at a recorded temperature of 21.6°C), found in a dense leaf litter near a creek situated at 920 m asl, within cloud forest in Henri Pittier National Park, Cordillera de La Costa (Aragua state) (vegetation sensu Huber and Alarcón 1988). A specimen MHNLS 17481 was found active during the night (21:00 h) on leaf litter. An additional individual not collected suggests that the species, or at least this population, seems active also overnight. At the same locality, a gravid female specimen was collected in August 2007, with an egg visible to the naked eye (specimen posteriorly liberated).
CONCLUDING REMARKS
Until now the clade is constituted by species with allopatric pattern and apparently restricted distribution, except Pseudogonatodes lunulatus that occurs in disjunct populations to the north of the Orinoco River, where it occupies different types of environments between 0-700 m asl (e.g. Andean submontane forest, shrublands and thorn bushes, semideciduous and deciduous forest) and P. guianensis a taxon widely distributed throughout the Amazon basin, mainly tropical humid forest below 300 m asl (cf. Duellman 1978, Vitt and De La Torre 1996, Gorzula and Señaris 1999, Molina 2001, Torres-Carvajal 2001, Doan and Arriaga 2002, ÁvilaPires 2005, Donnelly et al. 2005, Vitt et al. 2005). Despite we not found significant differences between populations of P. lunulatus, Ávila-Pires (1995: 30406) if found differences between populations of P. guianensis defining at least two phenetic groups, although she recognized that resolution requires better future evaluation.
Little is known about the ecological requirements of P. lunulatus that could contribute to understand its current distribution, but our data suggest occurring in moderate densities, being rather a cryptic species, active mainly daytime, semifossorial and/or cryptozoic. However, considering the habitat where the species is distributed, the same are largely fragmented or with different levels of transformation, being these mostly classified as "Endangered or Critically Endangered". For example, the deciduous forests occupy 6% of the territory, ~83% of its extension are suffering a medium or higher intervention, of which 14% has been lost completely, since their degree of transformation is high or very high. For its part, only 20% of these ecosystems are found in protected areas of conservation as National Parks (cf. Rodríguez-Morales et al. 2009, OliveiraMiranda et al. 2010). In this sense, preliminary extension is less than 100,000 km2, their habitats heavily impacted and disjunct populations, would be sufficient arguments to recognize this taxon in the category IUCN (2001) "Near Threatened".
Erroneously Rivas et al. (2012: 12) indicated to P. lunulatus as an endemic taxon of the country, ignoring the information given by Ávila-Pires and Hoogmoed (2000: 210), when they identified a specimen from Villavicencio, Meta department, eastern slope of Colombia as this taxon. In fact, we revised a specimen from the same locality (USNM 84970) that corroborates the information recorded by these last authors.
APPENDIX I: ADDITIONAL MATERIAL
Pseudogonatodes guianensis. MHNLS 1390313904. 5 km N Orinoco river, 2º14'35" N and 64º02'32"W. 410 m asl, col. César Molina, 24-25 October 1991, Río Negro municipality, Amazonas state; MHNLS 13980. Valley in Serranía Unturán, Río Negro municipality, Amazonas state, 200 m asl, col. César Molina.
Pseudogonatodes sp1. EBRG 3444. Quebrada Hoces 15 km E Puerto Píritu, 10º05' N and 64º53'W, col. Ramón Rivero, 10 March 1998, Anzoátegui state.
Pseudogonatodes sp2. CVULA 1775. El Palmar, 3 km SW La Soledad, col. J. Péfaur and J. Andrade, 06 October 1979, Bolívar municipality, Barinas state.
Pseudogonatodes cf. manessi. MIZA 0314. Trail to La Cumbre de Rancho Grande (1260 m), Parque Nacional Henri Pittier, Mario Briceño Iragorry municipality, Aragua state, Venezuela.
ACKNOWLEDGEMENTS
We would like to express our thank completely to Abraham Mijares-Urrutia, Colección Herpetológica Regional, Centro de Investigaciones en Ecología y Zonas Áridas (CIEZA), Universidad Francisco de Miranda; Francisco Bisbal and Ramón Rivero, Estación Biológica Rancho Grande (EBRG); Amelia Díaz de Pascual, Colección de Vertebrados, Universidad de Los Andes (CVULA); Celsa Señaris, Museo Historia Natural La Salle (MHNLS); Carlos Rivero-Blanco, Estación Ecológica Guáquira; David Kizirian, Lauren Vonnahme and David Dickey, Collection of herpetology, American Museum of Natural History, New York, EE.UU. (AMNH); Ronald Heyer, Jeremy Jacobs, James Poindexter and Roy McDiarmid, Collection of Herpetology, National Museum of Natural History, Smithsonian Institution, EE.UU. (USNM); Jonathan Losos, José Rosado and Jonathan Woodward, Museum of Comparative Zoology, Harvard University, Cambrige, EE.UU., (MCZ); Carol Spencer and Zima Bouzid, Collection of Herpetology, University of California, Los Angeles, EE.UU. (MVZ); Brown Rafe and Matt Buehler, Collection of Herpetology, Kansas University, Museum of Natural History, EE.UU. (KU); Steve Rogers, Carnegie Museum of Natural History, EE.UU. (CM) and Alan Resetar, Collection Manager Field Museum Natural History, Chicago, EE.UU., (FMNH). For its part, we thank to Jesús Manzanilla, Diego Cadenas, Francisco Navas, Enzo La Marca, Mariella Márquez, Douglas Mora, Milena Frontado, Dinora Sánchez, Mariset Medina, Ronald Luján and Lenin Reyes for their assistance in field or for Museum information. Finally, especially grateful to Carlos Laucho, Alejandro Morales, Damarys Grance and Vilma Savini for their logistical support during staying in the Biological Station Dr. Alberto Fernández Yépez (UCV).
REFERENCES
1.ATAROFF M, SARMIENTO L. 2003. Diversidad en Los Andes de Venezuela. I Mapa de unidades ecológicas del estado Mérida. CD-ROM, Ediciones Instituto de Ciencias Ambientales y Ecológicas (ICAE), Universidad de Los Andes, Mérida, Venezuela.
2. [ Links ]ATAROFF M, SARMIENTO L. 2004. Las Unidades Ecológicas de Los Andes de Venezuela. In: LA MARCA E, SORIANO P. (Ed). Reptiles de Los Andes de Venezuela, Fundación Polar, Conservación Internacional, CODEPRE-ULA, FUNDACITE Mérida, BIOGEOS, Mérida, Venezuela. pp. 12-26.
[ Links ]
3.ÁVILA-PIRES TC. 1995. Lizards of Brazilian Amazonia (Reptilia: Squamata). Zoologische Verhandelingen Leiden. 299:1-706. [ Links ]
4.ÁVILA-PIRES TC. 2001. A new species of Lepidopblepharis (Reptilia: Squamata: Gekkonidae) from Ecuador, with the redescription of Lepidoblepharis grandis Miyata, 1985. Occasional Paper of the Sam Noble Oklahoma Museum of Natural History. 11:1-11. [ Links ]
5.ÁVILA-PIRES TC. 2005. Reptiles. In: HALLOWELL T, REYNOLDS RP. (Eds.). Checklist of the Terrestrial Vertebrates of the Guiana Shield, pp. 25-40. Bulletin of the Biological Society of Washington. 13: 25-43. [ Links ]
6.ÁVILA-PIRES TC, HOOGMOED MS. 2000. On two new species of Pseudogonatodes Ruthven, 1915 (Reptilia: Squamata: Gekkonidae), with remarks on the distribution of some other sphaerodactyl lizards. Zoologische Medelingen Leiden. 73: 209-223. [ Links ]
7.BUSTILLOS SG. 1998. Atlas de Venezuela. Third Edition. Edit. Larense, Caracas, Venezuela, pp.315
8. [ Links ]DIXON JR, SOINI P. 1975. The reptile of the upper Amazon basin, Iquitos region, Peru. Part 1. Lizard and Amphisbaenians. Milwaukee Public Mus. Contrib. Biol. Geol. 4:1-58.
[ Links ]
9.DOAN TM, ARRIAGA WA. 2002. Microgeographic variation in species composition of the herpetofaunal communities of Tambopata region, Perú. Biotropica. 34:101-117. [ Links ]
10.DONNELLY MA, CHEN MH, WATKINS GG. 2005. Sampling amphibians and reptiles in the Iwokrama forest ecosystem. Proceeding of the Academy of Natural Sciences of Philadelphia. 154:55-69. [ Links ]
11.DUELLMAN WE. 1978. The Biology of an Equatorial Herpetofauna in Amazonian Ecuador. Miscellaneous Publications of the Museum of Natural History University of Kansas. 65:1352. [ Links ]
12.GARCÍA-PÉREZ JE. 1995. Una nueva especie de Ameiva bifrontata (Sauria: Teiidae) del bolsón árido de Lagunillas, Cordillera de Mérida, Venezuela. Rev. UNELLEZ Cien. Tec. 13(2):127-144. [ Links ]
13.GARCÍA-PÉREZ JE, YUSTIZ EE. 1995. Una nueva especie de Proctoporus (Sauria: Gymnophthalmidae) de los Andes de Venezuela. Rev. Ecol. Latinoam. 4:1-5. [ Links ]
14.GORZULA S, SEÑARIS CJ. 1999 “1998”. Contribution to the Herpetofauna of the Venezuelan Guayana I. A Database. Scientia Guaianae 8, Consejo Nacional de Investigaciones Científicas y Tecnológicas. Caracas, Venezuela.
15.HALLOWELL E. 1856. Notes on reptiles in the collection of the Academy of Natural Sciences of Philadelphia. Proc. Acad. Nat. Sci. Philadelphia. 8: 221-238.
16. [ Links ]HUBER O, ALARCÓN C. 1988. Mapa de Vegetación de Venezuela. Escala 1:2.000.000, Ministerio del Ambiente y de los Recursos Naturales Renovables y Fundación BIOMA, Caracas, Venezuela. [ Links ]
17.HUEY RB, DIXON JR. 1970. A new Pseudogonatodes from Perú with remarks on other species of the genus. Copeia. 3:538-642.
[ Links ]
18.KORNACKER PM. 1999. Checklist and Key to the Snakes of Venezuela. PaKo-Verlag, Rheinbach, Germany, pp. 270. [ Links ]
19.LA MARCA E. 1997. Lista actualizada de los reptiles de Venezuela. In: LA MARCA E. (ed.). Vertebrados Actuales y Fósiles de Venezuela.
[ Links ]
20. Serie Catálogo Zoológico de Venezuela. Vol. 1. Museo de Ciencia y Tecnología de Mérida, Venezuela, pp. 123-142. [ Links ]
21.LINNAEUS C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Laurentii Salvii, Holmiæ. 10th Edition, pp. 824. [ Links ]
22.LOTZKAT S. 2007. Taxonomie und Zoogeographie der Herpetofauna des Nirgua-Massivs, Venezuela. Frankfurt am Main, Germany: Johann Wolfgang Goethe-Univesität, Department of Biological Sciences [Thesis], pp. 160.
23.MANZANILLA J, FERNÁNDEZ-BADILLO A, VISBAL R. 1996. Fauna del Parque Nacional Henri Pittier, Venezuela: composición y distribución de los reptiles. Acta Cien. Venez. 47:191-204.
[ Links ]
24.MIJARES-URRUTIA A, ARENDS RA. 2000. Herpetofauna of estado Falcón northwestern Venezuela: a checklist with geographical and ecological data. Smithsonian Herpetological Information Service. 123:1-30.
25. [ Links ]MOLINA CR. 2001. Geographic distribution. Pseudogonatodes guianensis. Herpetol. Rev. 32:193. [ Links ]
26.NATERA MUMAW M, ESQUEDA GONZÁLEZ LF, CASTELAÍN FERNÁNDEZ M. 2015. Atlas Serpientes de Venezuela. Una Visión Actual de su Diversidad. Santiago, Chile, pp. 456. [ Links ]
27.OLIVEIRA-MIRANDA MA, HUBER O, RODRÍGUEZ JP, ROJAS-SUÁREZ F, OLIVEIRA-MIRANDA R, HERNÁNDEZ-MONTILLA M, ZAMBRANOMARTÍNEZ S. 2010. Riesgo de eliminación de los ecosistemas terrestres de Venezuela, pp. 109-225. En: RODRÍGUEZ JP, ROJAS-SUÁREZ F, GIRALDO HD. (Eds). Libro Rojo de los Ecosistemas Terrestres de Venezuela. Provita, Shell Venezuela, Lenovo Venezuela, Caracas, Venezuela, pp. 324. [ Links ]
28.PARKER HW. 1935. The frogs, lizards, and snakes of British Guiana. Proc. Zool. Soc. London. 105(3): 505-530. [ Links ]
29.PÉFAUR J, RIVERO JA. 2000. Distribution speciesrichness, endemism, and conservation of Venezuelan amphibians and reptiles. Amphib. Reptile Conserv. 2: 42-70. [ Links ]
30.RIVAS GA, MOLINA CR, UGUETO GN, BARROS TR, BARRIO-AMORÓS CL, KOK P. 2012. Reptiles of Venezuela: an updated and commented checklist. Zootaxa. 3211:1-64.
31. [ Links ]RODRÍGUEZ-MORALES M, CHACÓN-MORENO E, ATAROFF M. 2009. Transformación del paisaje de selvas de montaña en la cuenca del río capaz, andes venezolanos. Ecotropicos. 22(2):64-82. [ Links ]
32.ROUX J. 1927. Contribution à l’erpétologie du Vénézuéla. Verhandlungen der Naturforschenden Gesellschaft in Basel, 38:252-261.
33.RUTHVEN AG. 1915. Description of a new genus and species of lizard of the family Gekkonidae. Occasional Papers of the Museum of Zoology, University of Michigan. 19:1-3. [ Links ]
34.SHREVE B. 1947. On Venezuelan reptiles and amphibians collected by Dr. H. G. Kugler. Bulletin of the Museum of Comparative Zoology at Harvard College. 99:519-537. [ Links ]
35.TEST FH, SEXTON OJ, HEATWOLE H. 1966. Reptiles of Rancho Grande and vicinity, Estado Aragua, Venezuela. Miscellaneous Publications of the Museum of Zoology, University of Michigan. 128:1-63. [ Links ]
36.TORRES-CARVAJAL O. 2001. Lizards of Ecuador: Checklist, distribution and systematic references. Smithonian Herpetological Information Service, 131: 1-35. [ Links ]
37.VITT LJ, STELLA DE LA TORRE S. 1996. Guía para la Investigación de las lagartijas de Cuyabeno. Monografía 1. Museo de Zoología (QCAZ), Centro de Biodiversidad y Ambiente, Pontificia Universidad del Ecuador, Quito, Ecuador, pp. 166. [ Links ]
38.VITT LJ, SARTORIUS SS, ÁVILA-PIRES TC, ZANI PA, ESPÓSITO MC. 2005. Small in a big world: Ecology of leaf-litter geckos in new world tropical forests. Herp. Monographs. 19:137152. [ Links ]












 uBio
uBio 
