Boletín de Malariología y Salud Ambiental
versión impresa ISSN 1690-4648
Bol Mal Salud Amb v.47 n.1 Maracay ene. 2007
Evaluation of conventional serological tests for the diagnosis of American cutaneous leishmaniasis
Néstor Añez*, Agustina Rojas & Gladys Crisante
Investigaciones Parasitológicas J. F. Torrealba, Facultad de Ciencias, Departamento de Biología, Universidad de Los Andes, Mérida, 5101, Venezuela
*Autor de correspondencia: nanes@ula.ve
Three serological tests (ELISA, IFAT, DAT) were evaluated using sera from selected individuals with different American cutaneous leishmaniasis (ACL) clinical conditions. Reactivity in at least 2 of the 3 named tests was established as a criterion for declaring a patient as sero-positive. Prior to serological testing, people were diagnosed by clinical (presence of lesion = pl or scar=Sc), parasitological (presence of parasites =pp), immunological (LST) and molecular (PCR) methods. For a statistical comparison of the evaluated tests 4 groups of people were made up: 1) patients with active leishmanial lesion (n=44; pl, pp, +LST, +PCR); 2) patients who had recovered from leishmanial infection (n=43; Sc, +LST, +PCR); 3) asymptomatic individuals from endemic areas showing evidence of having contacted Leishmania-parasites (n=40; +LST, +PCR) and 4) Leishmania-negative people from the above localities where leishmaniasis is endemic and living under the same risk conditions, considered as healthy controls (n=104; -LST, -PCR). Considering the above-established criteria for sero-positives, the analysis of the results obtained with the 3 tests employed revealed very low sensitivity values. Seropositive figures of 50% were recorded for the first group, 9% for the second group, and 12.5% for the third group. Statistical analysis also revealed a low positive predictive value (PPV=0.73), a low negative predictive value (NPV=0.49), and a low kappa coefficient value (k=0.114). The present results raise questions about the use of the 3 conventional serological tests compared here to detect ACL at any clinical condition.
Key words: American cutaneous leishmaniasis, diagnosis, serology.
Evaluación de pruebas serológicas convencionales para el diagnóstico de la leishmaniasis cutánea americana
RESUMEN
Tres pruebas serológicas (TAD, IFI y ELISA) fueron evaluadas en sueros de pacientes con Leishmaniasis cutánea Americana (LCA) en diferentes condiciones clínicas. Para este propósito se estableció como criterio diagnóstico la reactividad de al menos dos de las tres pruebas para declarar un individuo seropositivo. Previo a la realización de la serología, los individuos seleccionados fueron diagnosticados clínicamente (presencia de lesión=pl o presencia de cicatriz =pc), parasitológicamente (presencia de parásitos =pp), inmunológicamente (IDR) y molecularmente (PCR). Para la comparación estadística de las pruebas serológicas evaluadas fueron conformados 4 grupos constituidos por: 1. Pacientes con lesiones activas (n=44; pl, pp, IDR +, PCR+); 2. Pacientes cicatrizados recobrados de una infección por Leishmania (n=43; pc, IDR+, PCR+); 3. Individuos asintomáticos de áreas endémicas con evidencias de haber tenido contacto previo conLeishmania (n=40; IDR+, PCR+) y 4. Individuos negativos controles de áreas donde la Leishmaniasis es endémica y quienes vivían bajo condiciones de riesgo (n=104; IDR-, PCR-). Los análisis estadísticos de los resultados obtenidos utilizando las tres pruebas serológicas indicadas, revelaron valores muy bajos de sensibilidad, siendo de 50%, 9% y 12% para los grupos de pacientes con lesión activa (1), pacientes recobrados de infección previa (2) e individuos asintomáticos (3) respectivamente, con una especificidad general de 89%. Asimismo, fue detectado un bajo valor predictivo positivo (PVP=0,73); un bajo valor predictivo negativo (PVN=0,49) y un muy bajo valor de coeficiente kappa (k=0,114). Los resultados obtenidos en el presente estudio arrojan dudas sobre la confiabilidad de las tres pruebas de diagnóstico serológico evaluadas aquí para detectar LCA en cualquier condición clínica.
Palabras claves: Leishmaniasis cutánea americana, diagnóstico, serología.
Recibido el 18/05/2006 Aceptado el 07/02/2007
INTRODUCTION
Leishmaniasis is a group of clinical manifestations recognized as cutaneous (CL), mucocutaneous (MCL) and visceral (VL) forms, produced as a consequence of the infection of susceptible people by parasites of the genus Leishmania (Kinetoplastida: Trypanosomatidae). This disease is among the more important public health problems facing populations worldwide, with an overall prevalence of 12 million people, an incidence of 2 million new cases occurring each year (1.5m CL and 0.5m VL), and with a 10th of the world´s population living under risk conditions in nearly 100 countries (WHO/TDR, 2002, Roberts et al., 2000). Its geographical distribution is determined by phlebotomine sandfly vectors, haematophagous insects in whose digestive tracts the parasite develops its infective forms which are transmitted to the vertebrate hosts during the bite.
American cutaneous leishnmaniasis (ACL) is an anthropozoonotic disease circulating in the New World from the south of the United States to the north of Argentina (Marques et al., 2001). It is caused by species of Leishmania grouped in the subgenera Viannia and Leishmania and transmitted by the bite of infected sand flies of the genus Lutzomyia (WHO, 2002). The infection in human population shows a variety of clinical pictures which range from localized, sometimes self-healing tegumentary lesion, to a much more aggressive deforming mucocutaneous lesion or to the diffuse form, although an inapparent infection is not uncommon (Rodrigues et al., 2002). The immune cellular response for the control of the Leishmaniainfection is related to the generation of helper T-cells by the host, which is able to deliver macrophage activating cytokines, particularly gamma interferon and interleukin-2 (Reiner & Locksley, 1992). In addition, the humoral immune response occurs during the active phase of the infection with the appearance of low titers of antibodies (Ab), which may disappear later (Behin & Jacques, 1984).
Serological methods have been used over the years in the diagnosis of ACL. However, the diagnostic value of the assay using crude antigens is considered to be limited because of their low reproducibility and specificity (Celeste et al., 2004). Despite this, there are serological tests still used in many diagnostic centers for detecting the prevalence of Leishmaniasis in endemic areas (Allain & Kagan, 1975). Among these the direct agglutination test (DAT), the indirect immunofluorescence antibody test (IFAT) and ELISA are some of the most commonly used tests. The present work deals with the evaluation of the abovementioned tests in groups of individuals at different ACL clinical conditions in order to compare them statistically to estimate their sensitivity and specificity and to reach conclusions about their reliability in the diagnosis of CL.
MATERIALS AND METHODS
Study groups
A total of 231 individuals (123 females and 108 males with a mean ± SD age of 28.2 ± 17.9 yr) was selected from 4351 people from different Venezuelan regions and were examined for ACL at the Parasitological Research Center, Faculty of Science, University of Los Andes, Merida, Venezuela. The selected samples were grouped as follows: Group 1 consisted of 44 patients (17 females and 27 males with a mean age of 29 yr) recently infected and suffering from active ACL. They were diagnosed by the presence of lesion (pl), the microscopical visualization of amastigotes forms (pp), leishmanin skin test positive (+LST), and positive skin biopsy and blood samples processed by Leishmania (Viannia)- specific PCR assay (+PCR). Group 2 consisted of 43 individuals (19 females and 24 males with a mean age of 33 yr) who had recovered from ACL as diagnosed by the presence of scar (Sc), +LST and positive blood sample PCR assay (+PCR). Group 3 consisted of 40 individuals (24 females and 16 males with a mean age of 28 yr) living in areas where Leishmaniasis is endemic who showed no clinical manifestation but were +LST and +PCR. Group 4 consisted of 104 healthy people (63 females and 41 males with a mean age of 25 yr) from the same areas indicated above and who also displayed negative LST (-LST) and negative PCR (-PCR). All individuals enrolled in the study provided written consent.
Sample collection and processing
Patient samples in active ACL cases Tissue scrapings or small 3-mm biopsy specimens were taken with a surgical blade at the border of the lesion under sterile conditions and local anesthesia with 2% lydocaine. Part of the recovered tissue was smeared onto a glass slide, fixed with methanol and stained with 10% Giemsa stain for microscopical observations. In vitro culture was carried out by placing a tissue fragment into tubes containing NNN medium. They were then incubated at 25ºC and weekly observed until 45 d. If positive they were transferred to fresh medium for maintenance and further work; otherwise, they were discarded. Either scraping or tissue fragments were also processed for PCR. Prior to the biopsy or lesion scraping, 5 ml of blood was collected by venipuncture from each patient. The serum was collected into two parts: the buffy coat was collected and used for PCR and hybridization assays, and the rest preserved at - 20ºC for serological analysis.
Leishmanin skin test (LST)
To asses the cellular immune response of individuals included in the different groups for the present study, a LST consisting of a promastigote antigen from resuspended Leishmania braziliensis was used. The soluble Leishmania antigen was prepared as described elsewhere (Reed et al., 1986). The LST was considered positive when indurations ≥ 5mm in diameter 48 h post injection were observed.
PCR and hybridization
Sera samples were processed for PCR using a Viannia subgenus specific primer set derived from L. braziliensis nontranscribed ribosomal gene spacer DNA sequences (Guevara et al., 1992) [GenBank accession M75133]. The details on the DNA from buffy coat of the sera samples regarding isolation and processing were given in a previous publication (Guevara et al., 1994). A repetitive sequence of 126 base pairs of ribosomal DNA of L. braziliensis was used as a PCR amplification target using the primers, forward 5´GCAGCACAGGGAAAG 3´; and reverse, 5´TACCTCTCTCCGTGATCG 3´. The reaction mixture (final volume, 25 μl) was prepared DNA amplifications were carried out in a Perkin-Elmer thermal cycler (Gene Amp PCR System 2400) as follows: an initial denaturation at 94ºC for 5 min, followed by 25 cycles of 94ºC, 52ºC, 72ºC of 1 min each, with a final extension at 72ºC for 5 min. All reactions were duplicated, and each set of reactions included a positive control consisting of 20 ng of purified DNA of Leishmania braziliensis, and a negative control containing no DNA. PCR products were visualized by electrophoresis in a 2% agarose gel stained with ethidium bromide. All the samples were hybridized with a L. braziliensis DNA probe labeled with digoxigenin (Roche Diagnostic GmbH- Mannheim, Germany) as previously reported (Guevara et al., 1994).
Serology
Serological methods used to detect circulating anti-Leishmania antibodies (Abs) included a direct agglutination test (DAT) pretreated with 2-mercaptoethanol (concentration of 0.5 OD crude antigen); an indirect immunofluorescence antibody test (IFAT) using 40 promastigotes/ 400X microscopical field and an enzyme-linked immunosorbent assay (ELISA)-(4μg/μl soluble protein). Tests were performed following standard procedures and adapted for detection of Leishmania Abs in our laboratory as previously reported (Añez et al., 2000). The equivalent dilution value for DAT and IFAT techniques to be considered as seropositive (cut-off titers) was that about 1:64 dilution, and for ELISA an optical absorbance ≥ 0.4±2SD. Patients were considered seropositive when they showed reactivity in at least 2 of the 3 serological tests.
Statistical analysis
This consisted of a comparison of serological tests (DAT, IFAT, ELISA) with other alternative diagnostic criteria considered to be highly reliable. These included clinical (presence of lesion), parasitological (detection of parasites), immunological (LST) and molecular (PCR) methods, defined above for each clinical condition. Comparison included estimation of sensitivity, specificity, positive predictive value, negative predictive value, observed agreement, expected agreement by chance, kappa coefficient with the standard error, Z statistic, and P value all at a 95 % of confidence interval. All these values were calculated by Epi Info 6.04.
RESULTS
Serological analysis in individuals with different ACL clinical conditions
From the 127 individuals selected to be examined by conventional serological tests to detect circulating specific Leishmania Abs at different ACL clinical conditions, 44 fulfilled the inclusion criteria to be considered as active cases (Group 1). They included 100% with pl, 88.6% (39/44) showing pp, 100% with + LST and 97% (38/39) showing +PCR for specific L. braziliensis DNA. The sera were examined using DAT, IFAT and ELISA methods. From the 44 sampled individuals, 20 (45%) revealed positive results in 2 of the 3 methods used, a condition established to consider a patient as seropositive. The analysis of the results with each particular serological test revealed a 9% seropositives when DAT was performed, 50% (22/44) with IFAT and 84% in the samples tested with ELISA test. Details of the obtained results of the serology of each single sample are shown in Table I. The analysis of the serological results obtained from the group made up by 43 individuals recovered from lesions at different time after infection (Group 2) revealed an overall percentage of seropositives of 9.3% (4/43) despite having 100% positive results with the diagnosis by LST and PCR (Table II). In this group values of 7%, 9% and 42% were recorded when DAT, IFAT, and ELISA methods were respectively performed (Table II). Similar results were detected in those asymptomatic individuals of the Group 3 bearing inapparent infection. In this case a 12.5% general sero-reactive was recorded and values of 15%, 8.0% and 30% were obtained with DAT, IFAT, and ELISA methods respectively (Table II).
Table I. Comparative methods for diagnosis of individuals with active ACL in Venezuela.
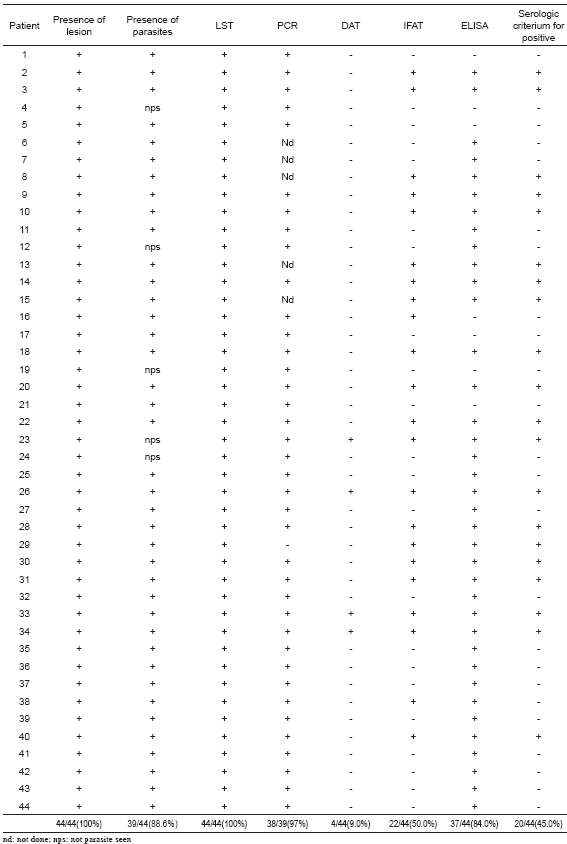
Table II. Compared sensitivity of three serological tests at different ACL clinical condition.
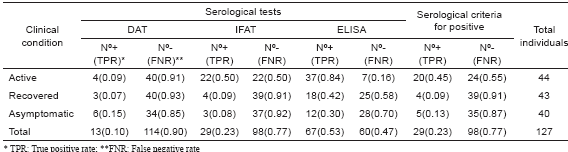
Statistical analysis
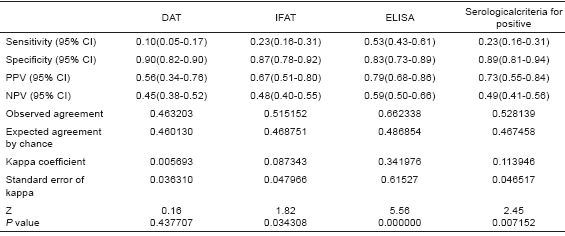
Statistical analysis showed that serological (TAD, IFAT and ELISA) methods used here to diagnose people at different ACL clinical conditions did not result in very reliable results, judging by the low concordance between the sero-test outcome and the established clinical condition group. The sensitive value expressed as true positive rate (TPR) considering G1-G3 and the 3 serological methods revealed an overall result of 0.23, with particular TPR of 0.45, 0.093 and 0.125 for G1, G2 and G3 respectively. In Table II details are given on the sensitive measures as TPR and FNR for 3 different serological tests and 3 ACL clinical conditions. Specificity, estimated as true negative rate (TNR), revealed more acceptable values, showing an overall TPR of 0.89 and values of 0.90, 0.87 and 0.83 for DAT, IFAT, and ELISA, respectively. Table III provides the statistical results used for comparison including PPV, NPV, Kappa coefficient, and p value.
Table III. Statistical comparison of serological tests used to diagnose ACL.
DISCUSSION
In the present study, from a total of 4351 people from different rural villages of western Venezuela examined for leishmaniasis, 231 were selected to evaluate conventional serological tests used to diagnose the cutaneous form of the disease. The chosen people selected were divided into 4 groups according to their clinical conditions. The first group (G1) was made up of 44 individuals suffering of active american cutaneous leishmaniasis (ACL), who were diagnosed by clinical (presence of active lesion), parasitological (presence of parasite at the lesion site), immunological (positive leishmanin skin test), and molecular (positive PCR) criteria. Another group (G2) consisted of 43 people who had recovered from ACL by the time they were sampled. Some of them had been diagnosed and treated at our laboratory, others in collaboration with the center for diagnosis and treatment of the Ministry of Health in Tovar, Merida, Venezuela. We were able thus to follow them during the course of the infection until they recovered. The Group 3 was made up of 40 people who, although living in areas where leishmaniasis is considered to be endemic, had never exhibited symptoms attributable to ACL according to their own testimony by the time of sampling. However, they showed positive results for LST and PCR. A fourth group (G4) of 104 healthy individuals also living in endemic areas as those of Group 3, but showing negative results for LST and PCR, were selected as the control group (G4).
The conformation of the selected study groups allowed us to have representative individuals with ACL at different clinical and immunological conditions. These circumstances appear to be ideal for comparing the product of the humoral response to the infection of Leishmania causing ACL using serological methods previously suggested as useful tools to detect circulating specific antibodies. Having created a statistically acceptable sample of the various groups and being able to use alternative methods to diagnose ACL at any clinical condition, we evaluated DAT, IFAT, and ELISA, 3 commonly used conventional serological methods, to asses its reliability as diagnostic tools. When all the clinical conditions and the diagnostic criteria established here to declare a seropositive (2 positive of the 3 tests used) were considered, an overall percentage of 23% was recorded, with values of sero-reactive of 45%, 9% and 13% for the clinical groups of active, recovered, and inapparent, respectively. When considering each particular serological test used to diagnose people with ACL at different clinical conditions, the examination also revealed low relative values for seropositives, including 10% for DAT, 23% for IFAT, and 53% for ELISA. This fact appears to indicate that theserological methods used in the present work under standard conditions do not offer confidence enough to be considered as reliable diagnostic tool for ACL. This assumption was particularly true when these serological tests were compared to other alternative methods recognized for their high specificity and sensitivity as LST, PCR, or the visualization of the parasite in active clinical cases (G1).
Under these circumstances, the present work demonstrated a remarkable inconsistence in the 3 serological tests used here to diagnose ACL. The fact that only a 45% of sero-reactive cases were detected compared to 88%, 97% and 100% for parasite detection, PCR and LST, respectively, appears to corroborate the above statement. In addition, statistical analysis of the results obtained using DAT, IFAT, and ELISA as serological tests also revealed a low positive predictive value (0.73), a low negative predictive value (0.49), and a low kappa coefficient value (k=0.114), indicating low concordance among them and a low level of reliability for considering these tests as recommendable tools for ACL diagnosis.
The results recorded in the present study appear to corroborate previous observations by other workers who reported that serological studies using whole serum may be considered of limited value in ACL because of the cross-reactivity and the remarkable different patterns of reactivity (Ulrich et al., 1988). Regarding sensitivity, none of the cases studied in the present work showed such a high value as to consider serology as a good diagnostic tool for ACL. This aspect appears to be a generalized problem for serology, and low values (63%) have also been reported for canine visceral Leishmaniasis (Ashford et al., 1995). The authors stated that such results in relation to sensitivity of serological tests raise questions about the use of serology to detect Leishmania-infections, suggesting that more sensitive methods as PCR might serve as a better gold standard to define infection by this parasite.
Finally, taking into consideration the various difficulties in establishing serological tests as reliable tools for ACL diagnosis, new or improved tools need to be produced. In order to be considered as an ideal indicator, a good serological tool must be simple, non-invasive, easy to centralize, cheap, and reliable. Meanwhile, validation of a new serological test for ACL and development of new primers for diagnosis and identification are required.
ACKNOWLEDGEMENTS
We are indebted to Dr. G. Fermín for carefully reviewing of the manuscript; Mr. N. Diaz and Mr. A. Fernandez for helping on the statistical analysis. This work was supported by projects sponsored by CDCHT-ULA and FONACIT-G- 2005000370 (complementary activities). The authors are grateful to REVELE for the very long discussion on the subject.
REFERENCES
1. Allain D. S. & Kagan I. G. (1975). A direct agglutination test for leishmaniasis. Am. J. Trop. Med. Hyg. 24: 232-236. [ Links ]
2. Añez N., Gonzalez C., Lugo de Yarbuh A., Gonzalez N., Rojas A., Crisante G., Percoco G., Guevara P. & Ramirez J. L. (2000). Association of trauma with Leishmania parasite recall. Clinical and experimental evidences. Bol. Dir. Malariol. San Amb. 40: 13-20. [ Links ]
3. Ashford D. A., Bozza M., Freire M., Miranda J. C., Sherlock I., Eulalio C., et al. (1995). Comparison of polymerase chain reaction and serology for detection of canine visceral leishmaniasis. Am. J. Trop. Med. Hyg. 53: 251-255. [ Links ]
4. Behin R. & Jacques L. (1984). Immune response to leishmaniasis. Crit. Rev. Trop. Med. 2: 141-188. [ Links ]
5. Celeste B. J., Angel S. O. & Castro L. G. M. (2004). Leishmania infantum heat shock protein 83 for the serodiagnosis of tegumentary leishmaniasis. Braz. J. Med. Biol. Res. 37: 1591-1593. [ Links ]
6. Guevara P., Alonso G., DaSilveira J. F., DeMello M., Scorza J. V., Añez N. & Ramirez J. L. (1992). Identification of New World Leishmania using ribosomal gene spacer probes. Mol. Biochem. Parasitol. 56: 15-26. [ Links ]
7. Guevara P., Rojas E., Gonzalez N.,Valera M., Scorza J. V., Añez N. & Ramirez J. L. (1994). Presence of Leishmania braziliensis in blood samples from cured patients or at different stages of immunotherapy. Clin. Diag. Lab. Immunol. 1: 385-389. [ Links ]
8. Marques M. J., Volpini A. C., Genaro O., Mayrink W. & Romanha A. J. (2001). Simple forms of clinical samples preservation and Leishmania DNA extraction from human lesions for diagnosis of American cutaneous leishmaniasis via polymerase chain reaction. Am. J. Trop. Med. Hyg. 65: 902-906. [ Links ]
9. Reed S., Badaró R., Masur H., Carvalho E. M., Lorenco R., Lisboa A., Teixeira R., Johnson W. & Jones T. C. (1986). Selection of a skin test antigen for American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 35: 79-85. [ Links ]
10. Reiner S. L. & Locksley R. M. (1995). The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13: 151-177. [ Links ]
11. Roberts L. J., Handman E. & Foote S. J. (2000). Leishmaniasis – Clinical Review. BMJ. 321: 801- 804 (bmj.com). [ Links ]
12. Rodrigues E. H., Felinto de Brito M. E. & Mendoca M. G. (2002). Evaluation of PCR for diagnosis of American cutaneous leishmaniasis in an area of endemicity in northeastern Brazil. J. Clin. Microbiol. 40: 3572-3576. 62 BOLETÍN DE MALARIOLOGÍA Y SALUD AMBIENTAL ACL serological diagnosis [ Links ]
13. Ulrich M., Centeno M., Mattout Z. & Convit J. (1988).Serological patterns and specificity in American cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 39: 179-184. [ Links ]
14. WHO/TDR. (2002). Special Programme for Research and Training in tropical disease. (2002.) Leishmaniasis. www.who.int/tdr. [ Links ]












 uBio
uBio 
