Boletín de Malariología y Salud Ambiental
versión impresa ISSN 1690-4648
Bol Mal Salud Amb v.49 n.2 Maracay dic. 2009
Genetic typing of Trypanosoma cruzi isolates from different hosts and geographical areas of western Venezuela
Néstor Añez1*, Gladys Crisante1, Néstor Añez-Rojas2, Agustina Rojas1, Glenda Moreno3, Flavia Maia da Silva4 & Marta M. G. Teixeira4
1 Investigaciones Parasitológicas "J. F. Torrealba", Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, 5101, Venezuela.
2 Grupo de Investigaciones en Biotecnología Aplicada (GIBA) Universidad Nacional Experimental Sur del Lago (UNESUR), Santa Bárbara de Zulia, Venezuela.
3 Laboratorio de Epidemiología Molecular, NURR-ULA, Trujillo, Venezuela.
4 Departamento de Parasitología, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, Brazil.
*Autor de correspondencia: nanes@ula.ve
A total of 72 Trypanosoma cruzi isolates from different hosts and geographical regions of western Venezuela, where Chagas disease is endemic, were typed using ribosomal and mini-exon gene markers. The isolates were obtained from wild, peridomestic and domestic sources including triatomine-bugs, human acute chagasic patients and other mammals. Results showed that T. cruzi two major phylogenetic lineages, T. cruzi I and T. cruzi II were present. However, a remarkable predominance of T. cruzi I (96%) over T. cruzi II (4%) was observed. The present results suggest that in western Venezuela circulation of both T. cruzi I and T. cruzi II isolates is independent from the source of isolation and the geographical area where they occur, with predominance of T. cruzi I. The epidemiological significance of the present results is discussed.
Key words: Trypanosoma cruzi, genetic typing, parasite sources, Chagas disease, Venezuela.
Tipaje genético de aislados de Trypanosoma cruzi de diferentes hospedadores y áreas geográficas del occidente de Venezuela
RESÚMEN
Un total de 72 aislados de Trypanosoma cruzi obtenidos de diferentes hospedadores y regiones geográficas del occidente de Venezuela, donde la enfermedad de Chagas es endémica, fueron caracterizados genéticamente utilizando marcadores moleculares de los genes ribosomales y del mini-exón. Los aislados fueron obtenidos de fuentes silvestres y domésticas, incluyendo triatominos-vectores, pacientes chagásicos agudos y otros mamiferos. Los resultados mostraron la presencia de los dos linajes reconocidos para T. cruzi (TcI y TcII) en la mayoría de los aislados provenientes de los diferentes hospedadores estudiados. Sin embargo, fue observada una marcada
predominancia de T. cruzi I (96%) sobre T. cruzi II (4%). Los presentes resultados sugieren que en el occidente de Venezuela la circulación de aislados de los linajes TcI y TcII es independiente de la fuente biológica de aislamiento y del área geográfica de procedencia, siendo predominante los aislados TcI. Se discute la significación epidemiológica de los presentes resultados.Palabras clave: Trypanosoma cruzi, caracterización genética, fuente de parásitos, enfermedad de Chagas, Venezuela.
Recibido el 13/05/2009 Aceptado el 11/09/2009
INTRODUCTION
Trypanosoma cruzi, the parasite causing Chagas disease (American trypanosomiasis), has been considered to be an organism exhibiting a pattern of clonal evolution. It comprises heterogeneous and highly polymorphic populations in their triatomine vectors, reservoir hosts and susceptible human groups living in risk areas where this parasite circulates. Despite their high genetic variability, T. cruzi populations have been classified into two major divergent phylogenetic groups or lineages (T. cruzi I and T. cruzi II) based on molecular markers (Souto et al., 1996; Zingales et al., 1998). The complexity of T. cruzi isolates make them differ in their genetic and biological properties, and consequently in their behavior in mammal hosts. In addition, these characteristics have been taken into consideration by several authors to associate the parasite variability with the different clinical profiles of Chagas disease, which may range from totally asymptomatic to severe or even fatal cases (Andrade, 1999; Macedo et al., 2004; Guhl et al., 2004, Añez et al., 2004; Zafra et al., 2008). Molecular epidemiology based on the genetic typing of T. cruzi isolates from different sources may be useful to understand the variability of this parasite and its relationship to clinical manifestation in chagasic patients. The present paper deals with the genetic typing of T. cruzi populations isolated from triatomine-vectors, mammal-hosts and human chagasic patients from rural localities of western Venezuela. With these results we attempted to shed some light on the controversial interpretation of T. cruzi-lineages (TcI-TcII) and parasite cycle (sylvatic-domestic) relationships and its association with clinical profiles in chagasic patients.
MATERIALS AND METHODS
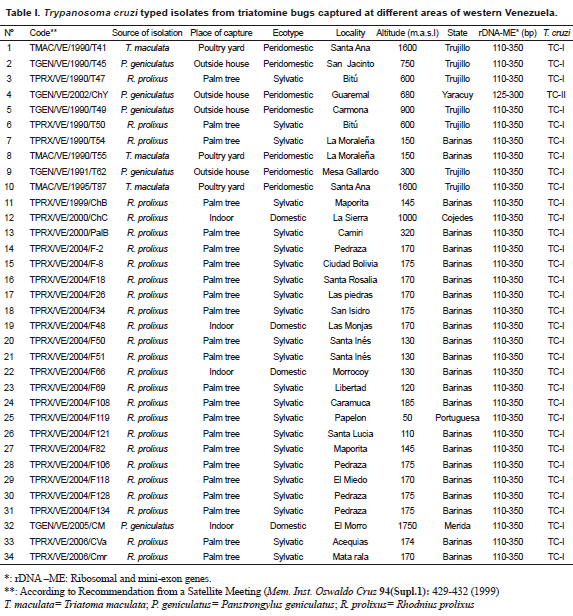
Origin, isolation, culture and genetic typing of Trypanosoma cruzi isolates Seventy two T. cruzi Venezuelan isolates were selected to be typed during the present work. They were separated into 3 groups according to the biological sources they were isolated from. A group of 34 isolates obtained from triatomine bugs including
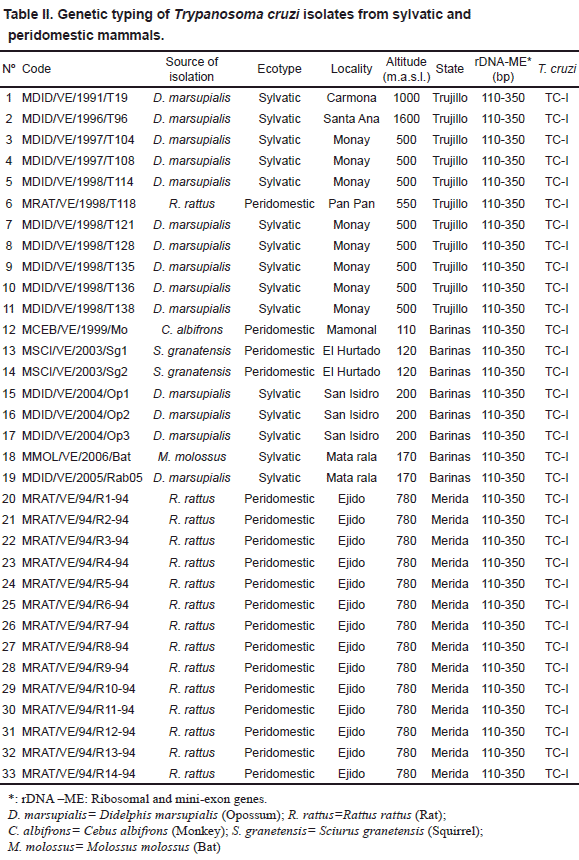
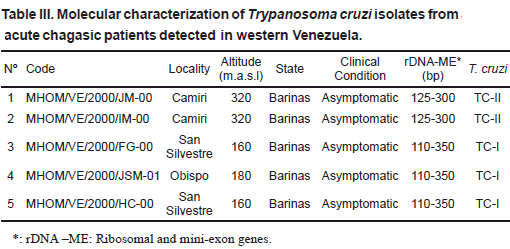
Rhodnius prolixus (26), Panstrongylus geniculatus (5) and Triatoma maculata (3) collected from palm trees (Attalea sp.=Scheelea sp. or Acrocomia sp.), poultry yards and houses (indoor or around) were used. The parasites were isolated from infected bugs, previously identified, which were dissected out, and the flagellates inoculated into young clean mice. Once the mice developed patent parasitemia, a sample of blood from each of the infected mouse was placed into tubes containing NNN culture medium. When established in the culture medium, the parasites were grown in mass, collected and used for genetic typing. Details on the code for each T. cruzi isolate, and the source of isolation, place of capture, ecotype and identification of bugs is given in Table I. In the second group, 33 isolates from different mammals were typed. This included isolates from opossum (14), rat (15), monkey (1), squirrel (2) and bat (1) sampled in 10 different localities. Sampling was performed by cardiopuncture and/or venopuncture. Blood samples were aseptically placed into culture tubes and processed as indicated above. Information on the isolates and details on isolation, are presented in Table II. The third group consisted of 5 T. cruzi isolates obtained from acute chagasic patients from 3 rural localities of the Barinas state (Table III). The parasites were isolated by hemoculture and processed as indicated above for the two other groups. In all cases a written consent was obtained from each sampled patient. This part of the work was approved by the scientific medical committee of the research council of the University of Los Andes, Mérida, Venezuela, and by the Biomedical Committee of the National Research Council in Venezuela.
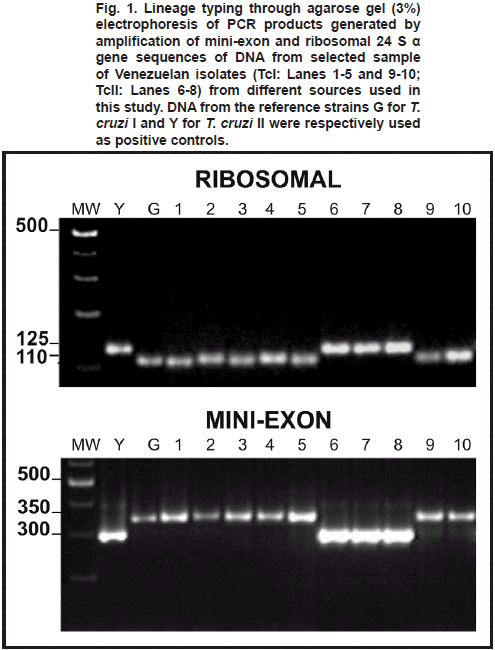
For the genetic typing of T. cruzi-isolates, DNA was extracted by the classical phenol-chloroform method. For polymerase chain reaction (PCR) amplification of the divergent domain of the 24 S α-ribosomal RNA gene, primers D71 and D72 were used as described by Souto et al. (1996). This method generated 110 bp (T. cruzi I-specific) or 125 bp (T. cruzi II-specific) DNA bands. For PCR amplification of an intergenic region of the mini-exon gene, a pool of primers TC, TC1 and TC2 were used following Souto et al. (1996), generating DNA bands of 350 bp or 300 bp for T. cruzi I or T. cruzi II, respectively. The PCR products were separated by electrophoresis in 3% agarose gel stained with ethidium bromide.
RESULTS
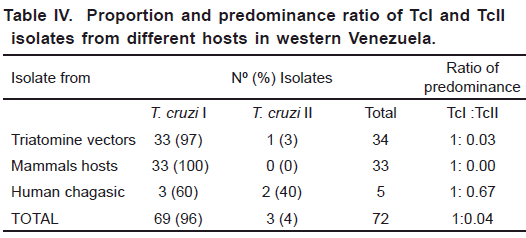
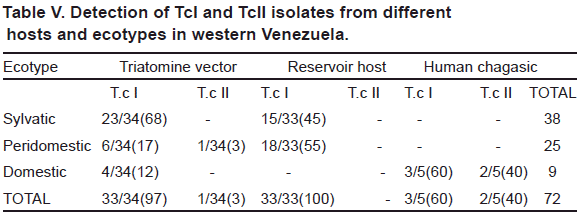
The genetic typing of 72 T. cruzi Venezuelan isolates from triatomine bugs (34), wild and peridomestic mammals (33) and acute chagasic patients (5), carried out by PCR amplification of DNA using the 24 α S- ribosomal and mini-exon genes, revealed the presence of both TcI and TcII lineages. Fig. 1 shows a selected sample of the lineage typing of isolates found during the study. In addition, results of the typing obtained for each particular isolate including origin, source of isolation, ecotype, locality and other geographical data are given in Tables I to III. The analysis showed TcI as the most frequent genotype found during this study (96%) being significantly predominant over Tc II (4%). This ratio of predominance was detected in all the study groups with a general difference of 1:0.04 (TcI:TcII). Details on the proportion of infection for any of the T. cruzi group including the ratio of predominance of TcI/TcII for the here studied hosts are presented in Table IV. Regarding lineages distribution, both TcI and TcII were present in hosts from different ecotypes with a remarkable predominance of TcI (Table V). The analysis of the clinical profile- T. cruzi
lineages association in isolates from acute chagasic patients also revealed the presence of both Tc I and Tc II, again with TcI predominating over TcII (Table III).DISCUSSION
The overall analysis of the present results, revealed the presence of both T. cruzi I and T. cruzi II lineages in the characterized isolates, with 96% of them typed as Tc I and the remaining (4%) identified as Tc II. This general trend was observed in all the studied isolates obtained from infected bugs, reservoir hosts and acute chagasic patients, showing a general ratio of predominance of TcI over TcII of 1:0.04. These findings confirmed the predominance of T. cruzi I populations in western Venezuela, a fact previously reported for this and other Latin-American countries (Coura et al., 2002; Añez et al., 2004; Carrasco et al., 2005; Herrera et al., 2007; Mejia-Jaramillo et al., 2009). This predominance is clearly observed in the typed isolates obtained from triatomine bugs in which 97% of them were recognized as belonging to TcI lineage, with only one isolate (3%) characterized as T. cruzi II. Considering the typing of parasites from bugs, TcI was detected in all the isolates obtained from R. prolixus and T. maculata, and in 4 out of the 5 characterized isolates from infected P. geniculatus, irrespective of its collection at the sylvatic (68%), peridomestic (17%) or domestic (12%) ecotypes. These findings seem to indicate that Venezuelan Tc I isolates circulate in any ecotype and may be successfully transmitted to vertebrates irrespective of the environment occupied by the species of bug acting as vector in a given endemic area. With these results it is hard to support that TcI is only associated with the sylvatic cycle, as previously suggested (Coura et al., 2002; Texeira et al., 2006; Aguilar et al., 2007). Regarding the detection of T. cruzi II in P. geniculatus, a suspected regional vector, our results also provide evidences on its circulation together with TcI in areas where Chagas disease is endemic. However, to establish epidemiological implications more investigation on isolates from a greater number of infected bugs is needed. Similarly, the genetic typing of T. cruzi isolates from mammal hosts also revealed the dominating presence of TcI from different species, ecotypes and geographical areas of western Venezuela a fact previously reported in domestic dogs from the same study area (Crisante et al., 2006). This study also revealed the wide distribution of the predominant TcI phylogenetic group, which was commonly detected in typed isolates obtained from animals sampled in sylvatic ecotypes (45%) as well as in peridomestic (55%) environments. Taken together our results seem to indicate that TcI may be indistinctly associated with sylvatic or domestic cycles and that this lineage is greatly responsible for the maintenance of infections in endemic areas of western Venezuela irrespective of the mammal host, ecotype, locality, altitude or any other geographical variable, supporting the above conclusion on the isolates from triatomine bugs.
The information gathered in the present work may serve to conciliate authors who have reported that TcI is associated with sylvatic cycle (Zingales et al., 1998; Coura et al., 2002; Aguilar et al., 2007) with those that, in contrast, have stated that TcI is associated with domestic cycle (Teixeira et al., 2006; Añez et al., 2004; Sousa et al., 2006).
Regarding T. cruzi lineages-clinical profile association in acute chagasic patients, the molecular typing of human isolates also revealed the presence of both TcI and TcII circulating at the same areas and provoking infections with similar clinical profiles, despite the predominance of TcI over TcII. This fact supports previous report detecting both of T. cruzi lineages (TcI –TcII) in asymptomatic individuals and in patients showing mild symptoms and severe heart failures, again with TcI provoking more severe cases than those infected with TcII (Añez et al., 2004). This fact also contradicts previous hypothesis by authors from the southern cone of South America who stated that severe clinical forms of Chagas disease occur primarily, or even only, in TcII-infected patients (Morel & Lazdins, 2003; Barret et al., 2003; Di Noia et al., 2002). Obviously, what appears to be a clear fact caused by TcII in the southern cone seems to be different in the northern part of South America including Venezuela and Colombia where both TcI and TcII lineages are associated with the pathology of Chagas´ disease having potential epidemiological implications (Añez et al., 2004; Zafra et al., 2008).
The results reported here disagree with those suggesting that prophylaxis and/or treatment of Chagas disease can be disregarded in regions where TcI but not TcII is found infecting people, and support Teixeira´s opinion that statement like this may result in dangerous conclusions for those countries, like Venezuela, where T. cruzi I is predominantly found infecting human (Teixeira et al., 2006).
Finally, the present results allow us to conclude that Chagas disease clinical profiles in human population of western Venezuela is variable, ranging from asymptomatic to severe, regardless of the, so far, known phylogenetic T. cruzi groups.
ACKNOWLEDGEMENTS
This work was supported by grant from FONACIT-G-2005-000370 and CDCHT-ULA-C-1209-03-A to NA. G. Crisante and A.Rojas are Ph.D students of the Biotechnology of Microorganism Post Graduate Program. N. Añez-Rojas is a Ph.D student of the Cellular Biology Post Graduate Program, at the Universidad de Los Andes. We are indebted to Prof. Dr. G. Fermín for carefully reviewing of the manuscript.
REFERENCES
1. Aguilar H. M., Abad-Franch F., Dias J. C., Junqueira A. C. & Coura J. R. (2007). Chagas disease in the Amazon Region. Mem. Inst. Oswaldo Cruz. 30: 47-56. [ Links ] 2. Andrade S. G. (1999). Trypanosoma cruzi: Clonal structure of parasite strains and the importance of principal clones. Mem. Inst. Oswaldo Cruz. 94 (Suppl. 1):185-187. [ Links ] 3. Anonymous (1999). Recommendations from a satellite meeting. Mem. Inst. Oswaldo Cruz. 94: 4. Añez N., Crisante G., Maia da Silva F., Rojas A., Carrasco H., Umezawa E. S. 5. Barrett M.P., Burchmare R.J.S. & Stich A. (2003). The Trypanosomiasis. 6. Carrasco H. J., Torrellas A., Garcia C., Segovia M. & Feliciangeli M. D. (2005). Risk of 7. Coura J. R., Junqueira A. C., Fernandes O., Valente S. A. & Miles M. A. (2002). Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 18: 171-176. [ Links ] 8. Di Noia J., Buscaglia C. A., De Marchi C. R., Almeida I. C. & Frasch A. C. C. (2002). A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas disease is due to a single parasite lineage. J. Exp. Med. 195: 9. Guhl F., Nicholls R., Montoya F., Rosas F., Velasco E., Herrera C. 10. Herrera C., Bargues M. D., Fajardo A., Montilla M., Triana O., Vallejo G. A. & Guhl F. (2007). Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect. Genet. Evol. 7: 11. Macedo A. M., Machado C. R., Oliveira R. P. & Pena S. D. (2004). 12. Mejia-Jaramillo A. M., Pena V. H. & Triana-Chavez O. (2009). 13. Morel C. M. & Lazdins J. (2003). Chagas disease. 14. Souto R. P., Fernandez O., Macedo A. M. & Campell 15. Teixeira M. M. G., Maia da Silva F., Marcili A., Umezawa E. S., Shikanai-Yasuda M. A., Cunha-Neto E. 16. Zafra G., Mantilla J. C., Magno-Valadares H., Mara-Macedo A. & González C. I. (2008). Evidence of 17. Zingales B., Souto R. P. & Mangia R. H. (1998). Molecular epidemiology of American tripanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. Int. J. Parasitol. 28: 105-112. [ Links ]












 uBio
uBio