Boletín de Malariología y Salud Ambiental
versión impresa ISSN 1690-4648
Bol Mal Salud Amb v.51 n.2 Maracay dic. 2011
Chagas disease inapparent infection in asymptomatic individuals from a Yukpa ethnic community in western Venezuela
Detección de infecciones inaparentes de la enfermedad de Chagas en individuos asintomáticos de la etnia Yukpa del occidente de Venezuela
Néstor Añez1*, Ricardo Atencio2, Zulbey Rivero3, Angela Bracho3, Agustina Rojas1, Maximiliano Romero1 & Gladys Crisante1
1 Investigaciones Parasitológicas "J.F.Torrealba", Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, 5101, Venezuela
2 Instituto de Investigaciones Clínicas "Dr. Américo Negrette", Laboratorio Regional de Referencia Virológica, Facultad de Medicina, Universidad del Zulia, Maracaibo, Venezuela
3 Escuela de Bioanálisis, Facultad de Medicina, Universidad del Zulia, Maracaibo, Venezuela
*Autor de correspondencia: nanes@ula.ve
SUMMARY
Inapparent infections by Trypanosoma cruzi were detected, for the first time, in symptomless seropositive healthy individuals from a Yukpa ethnic community in western Venezuela where Chagas disease had not been previously reported. Seropositivity was detected in 24 out of 173 (13.9%) sera samples taken from asymptomatic people by using three serological methods (DAT, IFAT, ELISA). Complementary analyses by IFAT revealed a low level of anti-T. cruzi specific IgM and IgG in all seropositive people when compared with detected levels in acute and chronic chagasic patients included as positive controls. In addition, 100% of the sampled dogs (8) were seropositive showing a level of anti-T. cruzi IgG similar to that detected in humans. Genotyping of a T. cruzi isolate obtained from an infected wild specimen of Rhodnius pictipes, revealed the circulation of lineage1 (DTU1) in the study area. The importance of the detection of asymptomatic T.cruzi-infections in this indigenous community and its potential epidemiological implications are discussed.
Key words: Chagas disease, Inapparent infection, Yukpa ethnic, Venezuela
RESUMEN
Infecciones inaparentes por Trypanosoma cruzi fueron detectadas, por vez primera, en individuos seropositivos asintomáticos muestreados en una comunidad Yukpa del occidente de Venezuela donde no se ha registrado hasta el presente enfermedad de Chagas. De un total de 173 muestras de suero examinadas 24 (13,9%) fueron registradas como seropositivas utilizando tres pruebas serológicas. Análisis complementarios por IFI revelaron bajos niveles de IgM e IgG cuando las muestras de individuos seropositivos asintomáticos fueron comparadas con casos agudos y crónicos incluidos como controles positivos. La totalidad de perros examinados (8) reveló seropositividad, registrándose niveles de IgG similares a los detectados en el grupo humano. La caracterización molecular de un aislado de T. cruzi obtenido de un espécimen silvestre de Rhodnius pictipes reveló la circulación del linaje 1 (DTU1) en el área de estudio. Se discute la importancia de infecciones asintomáticas por T. cruzi y sus implicaciones epidemiológicas en la comunidad indígena estudiada.
Palabras claves: Enfermedad de Chagas, Infección inaparente, Etnia Yukpa, Venezuela
Recibido el 25/08/2011 Aceptado el 03/10/2011
INTRODUCTION
Inapparent infections caused by Trypanosoma cruzi in areas where Chagas disease is endemic were first suggested by Garnham (1980), who foresaw its significance and pointed up that this stage may be considered in the general context of this disease. More recently, Añez et al. (2001) detected inapparent infections by T. cruzi in symptomless seropositive people living in close proximity to acute chagasic patients from rural areas of western Venezuela. The authors pointed out the significance of inapparent infections in Chagas disease taking into consideration the high proportion of seropositive people presenting this condition in the studied rural population. The above referred antecedents, together with the scanty information related to this subject, have motivated this investigation in a place where Chagas disease risk factors are suspected.
The present paper deals with the detection of inapparent infections by T. cruzi in asymptomatic seropositive healthy individuals from a Yukpa ethnic community in western Venezuela where Chagas disease had not been previously reported. The diagnosis was performed following serologic criteria using three different methods to detect seropositive people in the study population. In addition, estimation of T. cruzi-specific IgM/IgG levels in sera samples allowed to distinguishing those individuals bearing occult or inapparent infections in the indigenous community from the true acute or chronic positive control chagasic patients included for comparison purposes.
The detection of infected dogs and the presence of T. cruzi in a collected wild bug demonstrate circulation of the parasite in the study community and justify the present work.
MATERIALS AND METHODS
Study area
The present work was carried out in a local area, establishment of a Yukpa community named Toromo located at 275 m.a.s.l., between 72º 15´and 73º 15´W and 9º 00´ and 11º 10´ N at La Sierra de Perijá, state of Zulia in western Venezuela nearby the Colombian border. The village of Toromo (= Bijao leaf -Heliconia bihai- in Yukpa language) was established 40-50 years ago in a natural ecological system (N. Borjas & L. Martinez, 2011, personal communication). It presents an average annual rain fall of 1,500-2,400 mm, a mean temperature of 26ºC (range 30ºC - 13ºC) and a relative humidity over 70%. According to the information of the local health officer the village has at present nearly 1,000 inhabitants, living at different places of the community, named Nispero, Anapa, Manapa and Toromo. The main economic activities are agriculture and the manufacture of diverse tools made of plant materials.
Study population
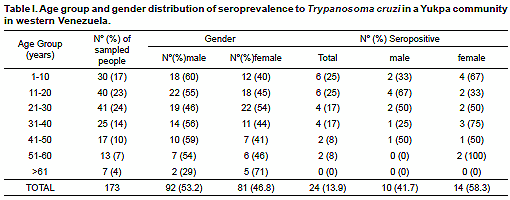
One hundred and seventy three healthy, asymptomatic individuals belonging to the Yukpa ethnic group were serologically evaluated during the present study. The group was composed of 92 males (53%) and 81 females (47%) with a male to female sex ratio 1:0.8, and a mean ± standard deviation age of 27.5 ± 17 years (range, 3 to 77 years).
Most of the evaluated people were able to recognize T. cruzi vectors when shown during an interview prior to sampling. They use to name the insect Shuparaku which they recognize as hematophagous; they also confirmed its presence indoor and in peridomestic areas. In addition, some of the interviewed people referred that they have occasionally eaten triatomine bugs and other insects (Konaiku). Regarding possible T. cruzi mammal hosts most individuals recognized Didelphis marsupialis, Dasipus novencintus, Cuniculus paca, among others, which they use to hunt and eat.
Sampled dogs
A total of eight dogs living in close proximity to seropositive individuals were sampled for serologic testing. Although present in almost all the houses, dogs resulted difficult to be sampled because they were extremely aggressive and the owners disliked animals´ disturbance. Sample consisted of 5mL of peripheral blood taken by venipuncture from the dog saphenous vein, and processed as indicated below.
Written consent from every single individual who entered the protocol, or from their representative in case of children, was obtained before the study plan and sample collection was carried out. The study was approved by the ethical committee at the Instituto de Investigaciones Clinicas, Faculty of Medicine, Universidad del Zulia, Maracaibo, Venezuela, and from a similar committee at Universidad de Los Andes, Mérida, Venezuela.
Sample collection and processing
A peripheral blood sample was obtained by venopuncture from each individual for parasitologic examination, PCR assay, and serologic testing. The two first observations were carried out using biphasic NNN culture medium and a protocol as previously reported (Añez et al., 1999a; Schijman et al., 2011). For serology, three serologic tests were used to detect circulating anti-T. cruzi antibodies (Ab). These included a direct agglutination test (DAT), an indirect immunofluorescence antibody test (IFAT) both for polyvalent and T. cruzi specific IgM/IgG subtypes, and an ELISA, following the conditions and procedures previously described (Añez et al., 1999a; 1999b; 2011). Samples from dogs were processed by DAT and by IFAT for specific T. cruzi IgG as previously reported (Crisante et al., 2006). In addition, parasites were isolated from an infected wild specimen of Rhodnius pictipes, collected in an open area of the village where members of the community were offering their products for sale. The wild bug was dissected out and abundant flagellates were detected in the rectal ampulla. A sample from the rectum was placed on a glass slide, fixed with methanol and stained with Giemsa stain, which revealed metacyclic forms morphologically similar to T. cruzi. Flagellates were used to inoculate mice, which exhibited blood circulating trypomastigotes 3 weeks later. Parasites were collected from an infected mouse by cardiopuncture and placed in an axenic diphasic NNN medium to grow a culture used for species identification and genetic typing of T. cruzi isolate by specific PCR assays as indicated below.
Diagnostic criteria
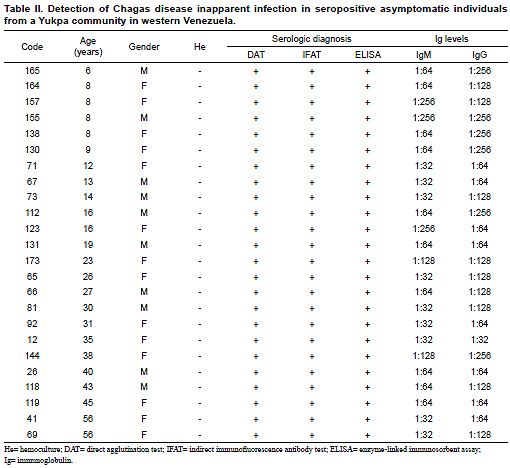
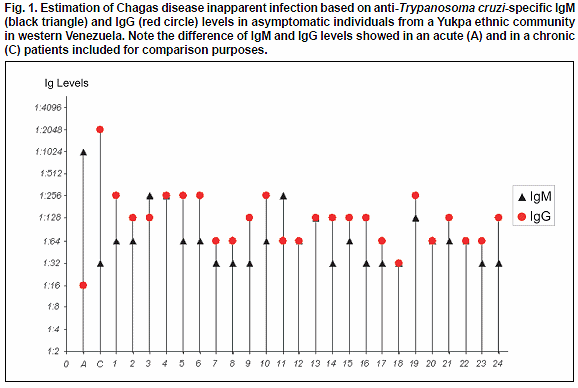
The diagnostic criteria used in this work were the same used in previous diagnosis to detect T. cruzi-seropositive individuals and/or for patients follow up (Añez et al., 2001). In all cases, sero-samples showing Ab titters ≥ 1:64 for DAT and IFAT and optical absorbance ≥ 0.4 for ELISA were considered positive for T. cruzi-infection. An individual was considered as seropositive when show reactivity in at least 2 of the 3 used serologic tests. Inapparent infections were considered those detected in asymptomatic seropositive people who showed by IFAT low IgM (≤ 1:256) and low IgG (≤ 1:256) levels as previously reported (Añez et al., 2001). This condition was established after contrasting them with samples from acute (≥ 1:512 IgM; ≤ 1:256 IgG) and chronic (≥ 1:512 IgG; ≤ 1:256 IgM) chagasic patients included as positive control for comparison purposes.
Parasite species identification and genetic typing by PCR assay
The identification of the isolate obtained from an infected wild bug was carried out demonstrating the presence of T. cruzi-DNA in samples taken from the established culture medium, using a specific PCR
assay for kDNA with primer:121(5´-AAATAATGTACGGGGGAGATGCATGA-3´)
and
122 ( 5´- GGTTCGATTGGGGTTGGTGTAATATA-3´).
PCR assay was performed following a protocol standardized by an international team work (Schijman
et al., 2011) and validated by WHO/PAHO (2008).In addition, the isolate was typed through PCR amplification using primers
D71 (5´- AAGGTGCGTCGACAGTGTGG-3´) and
D72 (5´- TTTTCAGAATGGCCGAACAGT-3´)
designed from the divergent domain of the 24 Sα RNA gene described by Souto et al.
(1996), and primersTC (5´- CCCCCCTCCCAGGCCACACTG-3´),
TC1 (5´- GTGTCCGCCACCTCCTTCGGGCC-3´)
and
TC2 (5´- CCTGCAGGCACACGTGTGTGTG-3´)
for amplification of an intergenic region of the mini-exon gene described by Souto et al. (1996). The PCR amplified product was separated by electrophoresis in
3% agarose gels, and stained with ethidium bromide.RESULTS
Serological analyses carried out in 173 individuals belonging to a Yukpa community from western Venezuela showed positive results for anti-
T. cruzi Abs in 24 (13.9%) of them. Except for the group over 60 years old, seropositives were detected in all the remaining study age groups regardless gender. During clinical examination the evaluated people did not show evidence of being suffering or have suffered Chagas disease. Although none of them recognized typical signs or symptoms attributable to T. cruzi-infection, most of the people were able to recognize triatomine-bug vectors (Shuparaku) as well as various mammal hosts as possible reservoirs of the parasite. Details on evaluated people including age group, gender and distribution of T. cruzi seropositivity is presented in Table I.The presence of anti-T. cruzi Abs was detected in healthy asymptomatic individuals with ages between 6 to 56 years old. Seropositivity was confirmed with the 3 serological methods used in each of the examined individual from the Yukpa community. In addition, using IFAT all the examined samples from seropositives revealed anti-T. cruzi specific IgM and IgG low levels with titers corresponding to dilutions between 1:32 to 1:256, which defined them as bearing T. cruzi inapparent infections according to the criteria established in the present work. No blood circulating trypomastigotes of T. cruzi were detected in any of the evaluated people when hemoculture and PCR assay were used to detect the parasite itself or portion of its genome. Table II shows details of the results obtained in the 24 seropositive individuals from the studied community including each serologic test used and the level of IgM/IgG detected during the analysis. Results of Ig (M and G) levels obtained in all the seropositive individuals were compared to those detected in the included acute and chronic chagasic patient positive controls, and are shown in Fig. 1.
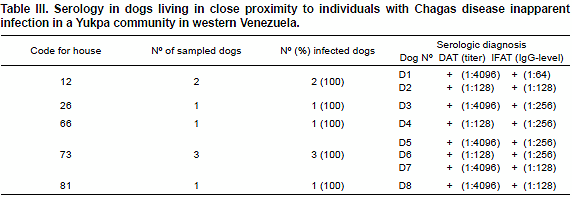
Similarly, examination of dogs living in close proximity to the above referred seropositive individuals also showed negative results for circulating parasites and positive results for anti-T. cruzi Abs. In all of them, high Ab-titters were detected according to the DAT test. However, IFAT analysis detected specific T. cruzi IgG at dilutions of 1:64 to 1:256 supporting result obtained in the human group. Details on the results in examined dogs are shown in Table III.
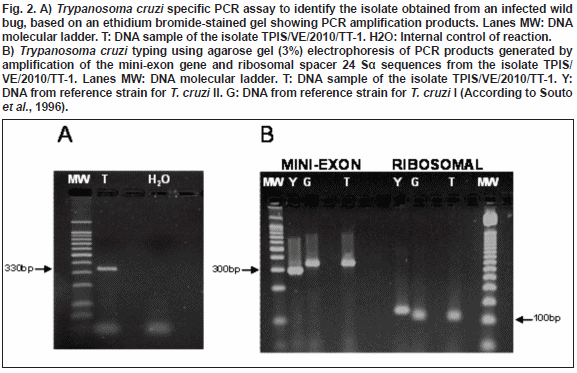
A T. cruzi isolate from a natural infected wild specimen of R.pictipes was obtained and named as TPIS/VE/2010/TT-1 following experts recommendation (Anonymous, 1999). The isolate was molecularly identified with a PCR assay specific for T. cruzi which amplified a fragment of 330bp from a kDNA minicircle variable region (Fig. 2A). Additionally, the isolate was genetically typed through a specific PCR amplification based on the divergent domain of the 24Sα RNA gene (D71/D72) and the intergenic region of the mini-exon gene (TC/TC1/TC2) generating 110bp and 350bp DNA bands for ribosomal and mini-exon genes, respectively, corresponding to T. cruzi DTU 1 (lineage I) as shown in Fig. 2B.
DISCUSSION
In the present study, from a total of 173 blood samples belonging to members of a Yukpa community serologically tested to detect circulating anti-T. cruzi Abs, 24 (13.9%) satisfied the established criteria to be considered as seropositives. The coincidence of the three serological methods used to diagnose the infected people, together with the physical healthy condition of the seropositive individuals confirmed during clinical examination, their testimony at the time of sampling they had never exhibited symptoms attributable to Chagas disease and the lack of previous report in the area of symptomatic chagasic patients, suggested the existence of T. cruzi inapparent infection in this indigenous community.
Although the sample size analyzed during this preliminary study seems to be insufficient to reach any conclusion on the significance of Chagas disease inapparent infections in this community, the interesting fact is that most of the infections were detected in the young population. In fact, from the 24 seropositive individuals detected during the sampling 25% were children under 10 years old, and a similar figure in the group up to 20 years old. These findings appear to support early ideas on inapparent infections proposed by Nicolle (1933), creator of the concept, who at the same time, stressed the importance to estimate to what degree this kind of infections affect children, since it should act as a potential genesis of an epidemic.
Clinical events attributable to Chagas disease in the study Yukpa community is, at present, unknown. However, risk factors detected here including the presence of triatomine-bugs and T. cruzi-isolates as well as infected animals and human hosts and excellent conducive environmental conditions for the development of the disease provide arguments to consider the region as potentially endemic for Chagas disease.
The fact that non acute and/or chronic cases have occurred during the last years may possibly be attributed to a special immunological condition present in the entire Yukpa population. This condition, together with a scanty number of parasites provoking infection after contacting with T. cruzi-infected wild bugs, may explain the presence of the here detected inapparent infection in the study community. The above argument seems to be supported by the excellent physical conditions observed in the screened people and the unsuccessful results obtained when houses were examined indoor looking for triatomine bugs. It is necessary to stress that the only bug collected from the study was a specimen of R. pictipes, which is basically a sylvatic species generally found in palm trees and in bromeliads ( Lent & Wigodzinsky, 1979). Although the collected bug showed a heavy infection, which allowed the isolation, identification and genetic typing of the circulating T. cruzi, the infrequent finding of the insect vector indoors may suggest another way to get the infection. This should be possibly attributed to infected domestic and/or wild mammal hosts. Suspicion for the former is based on all the examined dogs that resulted to be seropositives during the study, and for the latter, due to the common practice to hunt and eat wild animals, that highly probably were associated with infected bugs. In addition, as informed by local people, in this routine practice are involved dogs, hunters and all the family members who may infect themselves by establishing contact with parasites circulating in the blood, which is also frequently ingested without cooking it, in the belief that it cures some diseases (Shaman, 2011, personal communication).
Whatever the way infection is attained what appears to be important is the high proportion of seropositives detected in this indigenous community which uncover a nearly 14% seroprevalence, a figure significantly higher than the recorded average for areas where Chagas disease is endemic in Venezuela (Añez et al., 2003; 2004; Feliciangeli et al., 2003; Suarez et al., 2004; Sanchez-Martin et al., 2006).
More impressive seems to be the fact that 25% of the 24 infected individuals were children from 6 to 9 years old, who were strongly reactive in all the serological methods used for diagnosis, showing all of them IgG levels between 1:128 and 1:256 which represent 40% (6/15) of the seropositive people bearing the highest titters for this T. cruzi specific Ig. These figures are reinforced by the fact that 20% of the screened children under 10 showed to be infected, a result also detected in the group from 11 to 20 years which revealed a 15% (6/40) of infection.
These findings seem to indicate that T. cruzi infection is, at present, an active event in the Yukpa community judging by its frequent appearance in the young population, which appears to have a special resistance to the parasite since they do not show the typical symptoms related to Chagas disease observed in other endemic areas (Añez et al., 1999a; 1999b). However, a much more detailed study is suggested in order to establish the cause provoking the asymptomatic clinical profile and the low threshold humoral response in the T. cruzi seropositive people detected during the present work.
The examination of dogs living in close proximity to members of the community that resulted seropositives to T. cruzi bearing Chagas disease inapparent infection, served to evaluate the role of these animals as a potential risk factor in the maintenance of the parasite in the study area. Indeed, the fact that 100% of the dogs sampled in 5 of the houses showed to be seropositive appears to indicate that this mammal should be incriminated in the circulation of T. cruzi in the yukpa community acting as a source for infection to human, as previously reported in areas where Chagas disease is endemic (Zeledon et al., 1973; Gürtler et a.., 1996; Crisante et al., 2006).
One more point to be added to the aforementioned facts that may have potential epidemiological importance, is the finding of the circulation of T. cruzi lineage I (DTU1) carried by R. pictipes, a species that so far has not been incriminated in the transmission of Chagas disease in Venezuela.
Finally, the results obtained in this preliminary study, detecting for the first time Chagas disease inapparent infection in the Yukpas, may serve to call the attention for the potential epidemiological problem in this indigenous community, and also suggest that more investigation must be done in all aspects concerning this parasitic disease.
CONFLICT OF INTERESTS: None declared.
ACKNOWLEDGEMENTS
This work was supported by grant from FONACIT-G-2005-000370 (N.A.). G. Crisante and A. Rojas are Ph.D. students of the Biotecnology of Microorganism Post Graduate Program, Universidad de Los Andes-Mérida, Venezuela. A. Bracho is a Ph.D. student of the National Parasitology Post Graduate Program, Universidad Central de Venezuela, Caracas-Venezuela. We are indebted to Prof. G. Fermin for carefully reviewing the manuscript. This modest contribution is dedicated to the people of Toromo.
REFERENCES
1. Anonymous (1999). Recommendations from a satellite meeting. Mem. Inst. Oswaldo Cruz. 94: 429-432. [ Links ] 2. Añez N., Carrasco H., Parada H., Crisante G., Rojas A., González N., et al. (1999a). Acute Chagas Disease in western Venezuela: A clinical, seroparasitologic, and epidemiologic study. 3. Añez N., Carrasco H., Parada H., Crisante G., Rojas A., Fuenmayor C., 4. Añez N., Crisante G., Rojas A., Carrasco H., Parada H., Yepez Y., 5. Añez N., Crisante G., Rojas A., Diaz N., Añez-Rojas N., Carrasco H., Parada H. 6. Añez N., Crisante G. & Rojas A. (2004). Update on Chagas Disease in Venezuela - A Review. 7. Añez N., Crisante G., Caraballo F., Delgado W. & Parada H. (2011). 8. Crisante G., Rojas A., Teixeira M. M. G. & Añez N. (2006). Infected dogs as a risk factor in the transmission of human 9. Feliciangeli M. D., Campbell-Lendrum D., Martinez C., Gonzalez D., Coleman P. & Davis C. (2003). Chagas disease control in Venezuela: Lessons for the Andean region and beyond. 10. Garnham P. C. C. (1980). The significance of inapparent infections in Chagas disease and other forms of trypanosomiasis. Mem. Inst. Oswaldo Cruz. 75: 11. Gürtler R. E., Cecere M. C., Castanera M. B., Canale D., Lauricella M. A., Chuit R. & Segura E. L. (1996). Probability of infection with 12. Lent H. & Wygodzinsky P. (1979). Revision of the Triatominae (Hemiptera:Reduviidae) and their 13. Nicolle C. (1933). Les infections inapparentes. 14. Sanchez-Martin M. J., Feliciangeli M. D., Campbell-Lendrum D. & Davis C. R. (2006). Could the Chagas disease elimination programme in Venezuela be compromised by reinvasion of houses by sylvatic Rhodnius prolixus bug populations? 15. Schijman A.G., Bisio M., Crisante G., Añez N., 16. Souto R. P., Fernandes O., Macedo A. M., Campbell 17. Suárez B., Hernández M., Duque N., Martínez C. & Feliciangeli M. D. (2004). Conocimiento sobre la enfermedad de Chagas en los estados Barinas y Portuguesa, Venezuela. Bol. Mal. Salud Amb. 44: 109-118. [ Links ] 18. WHO/PAHO (2008). In: Schijman, A. (Ed.) Workshop on standardization and validation of clinical use of PCR for Trypanosoma cruzi DNA detection in Chagas disease 19. Zeledon R., Solano G., Zúñiga A. & Swartzwelder J. C. (1973). Biology and ethology of Triatoma dimidiata (Latreillie, 1811). III. Habitat and blood sources.












 uBio
uBio