Boletín de Malariología y Salud Ambiental
versión impresa ISSN 1690-4648
Bol Mal Salud Amb v.51 n.2 Maracay dic. 2011
Trypanosoma cruzi primary infection prevents severe re-infection in mice
Primoinfección por Trypanosoma cruzi previene re-infecciones severas en ratones
Néstor Añez*, María Luisa Martens, Maximiliano Romero & Gladys Crisante
Investigaciones Parasitológicas "J.F.Torrealba", Universidad de Los Andes, Facultad de Ciencias, Departamento de Biología, Mérida, 5101, Venezuela
*Autor de correspondencia: nanes@ula.ve
SUMMARY
The effect of Trypanosoma cruzi re-inoculations on experimentally infected mice was evaluated. Mice received a primary infection by intradermal inoculation of 5x103 T. cruzi metacyclic infective forms from laboratory infected Rhodnius prolixus. From 200 mice initially infected, 52 survived the course of the infection during 23 weeks. From these, 45 mice were re-inoculated and seven used as infected control. Two re-inoculations were performed using the same conditions as in the prime-infection. Observations on re-inoculated mice revealed a parasitemia level lower than that detected during the primary-infection. Serologic evaluation showed no variation in the immunoglobulin profile, maintaining similar IgM and IgG levels after re-inoculations. Similar mortality rates were observed in primary-infected, re-inoculated and infected control mice. No remarkable histopathological changes attributable to re-inoculation were detected. These results lead us to conclude that T. cruzi re-inoculation in mice previously infected with the same parasite does not produce a reactivation of infection similar to the typical acute clinical or immunological profiles. This also suggests that T. cruzi primary infection may prevent severe re-infection, establishing a protective stage making infected mice resistant to a second infection. Epidemiological implications of the present findings are discussed.
Key words: Trypanosoma cruzi, Primary-infection, Re-infection, Chagas disease.
RESUMEN
Se evalúa el efecto de reinoculaciones por Trypanosoma cruzi en ratones experimentalmente infectados. De un total de 200 ratones que fueron infectados por vía intradérmica con un inóculo de 5x103 tripomastigotes metacíclicos provenientes de especimenes de Rhodnius prolixus, 52 sobrevivieron a la primo-infección luego de 23 semanas. De estos 45 fueron re-inoculados en la misma forma como en la infección primaria y siete fueron utilizados como controles infectados no re-inoculados. Observaciones sistemáticas llevadas a cabo en muestras de los ratones re-inoculados revelaron parasitemias significativamente menores que las detectadas durante la primo-infección. La evaluación serológica no mostró diferencias en los niveles de IgM e IgG entre ratones primo-infectados y los re-inoculados. Observaciones histopatológicas no mostraron cambios atribuibles al efecto de las re-inoculaciones en muestras de corazón y músculo esquelético. Una tasa similar de mortalidad fue observada en ratones primo-infectados, re-inoculados y controles infectados. Se concluye que re-inoculaciones con T. cruzi en ratones previamente infectados con el mismo parásito no producen reactivación de la infección similar al típico cuadro agudo. Asimismo, se sugiere que la primo-infección por T. cruzi previene una reinfección severa estableciendo un estado de protección y resistencia a subsecuentes infecciones. Se discuten las implicaciones epidemiológicas de los presentes hallazgos.
Palabras claves: Trypanosoma cruzi, Primo-infección, Re-infección, Enfermedad de Chagas
Recibido el 21/07/2011 Aceptado el 19/10/2011
INTRODUCTION
The possibility of reinfection by Trypanosoma cruzi in chagasic patients has always been an unsolved question, which have made difficult to quantify the actual incidence of acute cases in areas where Chagas disease is endemic. This is troublesome due to the risks to which are submitted people living in endemic areas constantly exposed to new T. cruzi-infections. This fact has been suggested as one of the main variables responsible for the severe clinical alterations detected during the evolution of T. cruzi chronic infection (Silveira, 2000; Bustamante et al., 2002, 2003). However, no accurate information on the phenomenon is yet available as to express any definite opinion about it and more investigation on the subject is urgently needed. In this context, different authors have shown that re-inoculations to previously T. cruzi-infected individuals do not produce significant changes on the parasitemia level and mortality rate of experimental animals (Brener, 1962; Menezes, 1969; 1970; 1971; Revelli et al., 1990). Nevertheless, other authors have shown that T. cruzi re-infections determine the severity of cardiac damage in mice, observing higher parasitemia levels in re-infected than in infected animals (Bustamante et al., 2002).
It is well known that once succeeded the acute phase of Chagas disease the host immune system is able to control the parasite proliferation through mediating mechanisms as the humoral response, that is able to keep in check and in low levels the amount of blood circulating trypomastigotes. In addition, the cellular response, although always present during the course of the infection, is unable to totally eliminate the intracellular amastigote forms which may persist in small foci in the heart and other tissues of the host during the chronic phase (Dos Reis & Lopes, 2000; Añez et al., 1999b; 2011). Despite the abundant information supporting the above statement, the apparent resistance to T. cruzi- reinfection is, so far, a poorly studied phenomenon.
The present work deals with the effect of T. cruzi re-inoculation in experimentally infected mice which had succeeded a primary infection. This was assessed by evaluating the course of infection and re-inoculation using parasitological, serological, histopathological and molecular (PCR) methods, hoping to shed some light on the controversial interpretation of T. cruzi re-infection in the mammal host.
MATERIALS AND METHODS
Parasites
Metacyclic infective forms of T. cruzi, isolate MHOM/Ve/1992/YBM2-92, were obtained from experimentally infected Rhodnius prolixus kept under laboratory conditions. The T. cruzi isolate belongs to the lineage I [=DTU I] (Añez et al., 2004; Zingales et al.
, 2009) and was isolated from a severe case of acute Chagas disease detected in an endemic area of western Venezuela, maintained in the laboratory by passages mice-bugs and used to infect and re-inoculate mice. Recovered metacyclic trypomastigotes were observed under a light microscope for activity, and for morphology estimation in a Neubauer haemocytometer chamber (T. cruzi metacyclic-forms/mL) and kept in PBS until used.Infection
A total of 200 albino male (30gr, 30d old) NMRI out-bred mice were intradermically inoculated with 5x103 metacyclic infective forms of the above T. cruzi isolate. Observations on the course of the infection began one week post-inoculation (w.p.i.). Once a week, during 23 weeks, seven infected mice were discontinuously sampled taking them at random from the total group to estimate the average parasitemia (T. cruzi-blood trypomastigotes/mL) using blood samples from the tail. From the seven sampled infected mice, two of them were weekly selected and processed to observe the effect of the primary-infection at different times. They were anaesthetized and then sampled for parasitological, serological, histopathological and molecular (PCR) evaluation by the methods described below. Mortality in the group of experimentally T. cruzi-infected mice was recorded by daily observation until the end of the experiments.
Re-inoculation
From 200 initially T. cruzi-infected mice, 102 died during the 23 weeks of the infection and 46 were killed for weekly observation, surviving 52 of them. From these, 45 mice were re-inoculated at week 33 using the same number of parasites and via of inoculation than those used to produce the prime-infection. The other seven survivor mice were used as infected control, receiving no re-inoculation. Re-inoculation was performed after repeated microscopic examination detecting no blood-circulating parasites in the survivor mice. A second re-inoculation under the same conditions was performed at week 39 from the beginning of the experiment. Evaluation of the course of re-inoculation was carried out by weekly sampling of both re-inoculated and infected control mice in the way indicated for the course of prime-infection.
Sample collection and processing methods used to evaluate the course of T. cruzi infection and re-inoculation in mice To detect and evaluate the course of parasitemia level during the T. cruzi
primary infection and re-inoculation in the experimental animals, qualitative and quantitative parasitological methods were used. These included fresh peripheral blood samples and blood smears stained with 10% Giemsa stain in phosphate buffer, pH 7.2, for microscopic examination; hemoculture of 0.1 mL of blood in NNN culture medium; and xenodiagnosis using 5 IV instar nymphs of R. prolixus from a closed colony kept in the laboratory. Details for each of the examination methods have been published elsewhere (Añez et al., 1999a). The course of parasitemia estimation (blood trypomastigotes/mL) in the experimental mice was carried out using a Nebauer haemocytometer chamber filled with 5 μL of blood taken from the tail of each infected mouse. Serological evaluation of the evolution of circulating anti-T. cruzi antibodies during the prime infection and successive re-inoculations was performed using an ELISA assay. Sero-samples showing an optical absorbance (OA) ≥ 0.4 were considered positive for T. cruzi-infection. Conditions and procedures for the serological method have been described elsewhere (Añez et al., 2001).The histopathological evaluation was carried out on samples from the heart and skeletal muscle of weekly selected mice during the course of the
T. cruzi prime infection and/or re-inoculations. The Giemsa-colophonium and hematoxylin-eosin (H-E) staining techniques for histological sections and two other immunohistochemical methods were used to visualize the distribution and amount of T. cruzi intracellular form in the studied tissues. The latter techniques included an indirect immunofluorescence test (IIT) for detection of T. cruzi antigenic deposit in tissue samples, and the peroxidase anti-peroxidase (PAP) method to specifically visualize T. cruzi amastigotes in the same tissues. Details for the protocol and sample examination of the above mentioned methods have been previously provided (Añez et al., 1999b).To complement observations with the above indicated methods a specific
T. cruzi kDNA-PCR assay using primers 121 (5´-AAATAATGTACGGGGGAGATGCATGA-3´) and 122(5´-GGTTCGATTGGGGTTGGTGTAATATA-3´) was performed in skeletal muscle and heart samples of experimental animals following previous reported methodology (Schijman
et al., 2011; Añez et al., 2011).The experiments were ethically conducted according to the approved guide for the care and use of laboratory animals at the Central Animal House of Universidad de Los Andes, BIOULA, Merida, Venezuela.
RESULTS
Parasitological evaluation
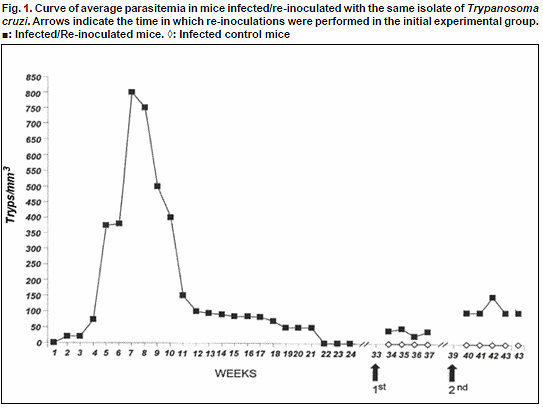
Weekly estimation of the parasitemia level in T. cruzi-primary infected mice revealed at the beginning of the infection a scanty number of blood trypomastigotes showing an average infection < 50 parasites/mm3 in 14-29% of the sampled mice. From the 4th week onwards an increase of the parasitemia was detected, which reached the highest level during the period between 7-10 w.p.i with a maximum average of 800 tryp/mm3 and values in some samples over 1000 tryp/mm3. During this period 86-100% of the sampled mice showed blood circulating trypomastigotes. Parasitemia started to decrease from the 11th week, a trend observed until 21w.p.i. From this period, over 50% of the sampled animals showed parasitemias under the level of detection of the used method. On weeks 22 and 23 no circulating parasites were microscopically seen, a fact observed until week 33 p.i. (Fig. 1). This observation allowed us to consider the remaining animals as survivors from a T. cruzi
-primary infection, having been submitted to an experimental re-inoculation.Estimation of the parasitemia level in mice after the first re-inoculation, revealed the presence of blood trypomastigotes in 14-43% of the sampled mice, showing < 50 tryps/mm3 during 4 weeks of observation. In all the samples the estimated parasitemia was significantly lower than that observed in mice during the course of T. cruzi primary infection (Fig.1).
Two weeks later (39 w.p.i) a second re-inoculation was performed in the remaining survivor mice. Weekly observations revealed that during the
first three weeks following the 2nd re-inoculation most of the sampled mice showed no-circulating parasites (≥60%) detecting a low active parasitemia in 28-40% of them, with blood trypomastigotes fluctuating around 100 tryps/mm3. As observed during the first re-inoculation, in the second time re-inoculated animals parasitemia was maintained in levels significantly lower than the observed during the course of the primary infection. Details on the average parasitemia detected in T. cruzi infected, re-inoculated and control mice are presented in Fig.1.To complete the parasitological evaluation mice were discontinously examined during the course of primary-infection (46) and successive re-inoculations (9) using xenodiagnosis, Giemsa stained blood smears and hemoculture, resulting xenodiagnosis the most sensible methods detecting 86% and 100% of infected mice during the primary T. cruzi-infection and re-inoculations, respectively.
Serological evaluation
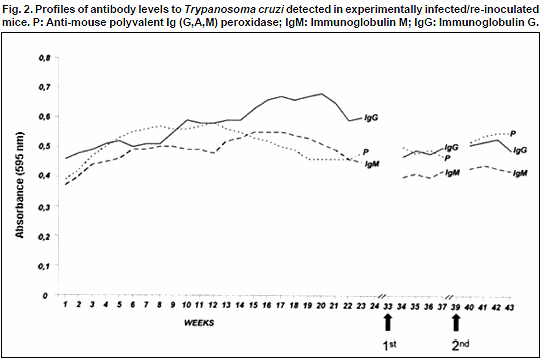
Circulating anti-T. cruzi antibodies (Ab) were detected from the 3rd w.p.i. in concordance with the increase of parasitemia. However, when parasitemia fell under the level of detection according to the results of microscopic observations, the serological test revealed the same Ab levels (OA: 0.4-0.5) demonstrating a well established humoral response in the infected mice. Observations carried out after the first (34-37w.p.i.) and the second (40-43w.p.i.) re-inoculations, revealed no-significant variation in the immunoglobulin (IgM/IgG) profile detected in the re-inoculated groups (OA: 0.4-0.5). Details on the evolution of circulating Ab level in T. cruzi-infected and re-inoculated mice are shown in Fig.2.
Histopathological evaluation
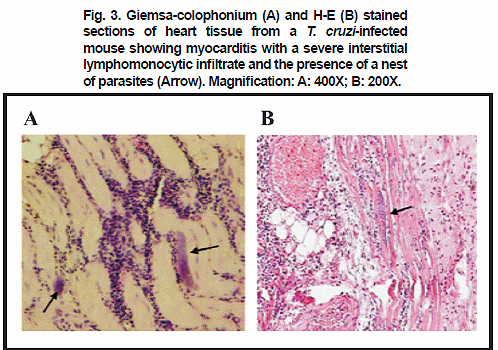
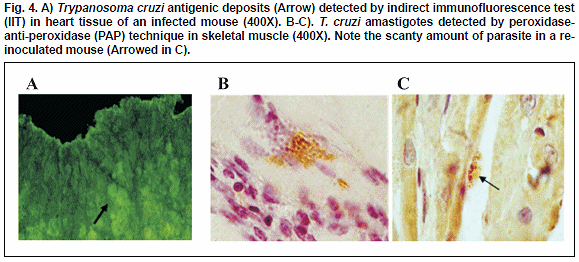
Intracellular T. cruzi amastigotes pseudocysts were consistently detected in Giemsa-colophonium and H-E stained sections of heart and skeletal muscle of infected mice over the 4th week of the course of the prime-infection. In both infected tissues inflammatory infiltrates were detected with mononuclear predominance and lymphocyte abundance from the 7th w.p.i. (Fig.3). The immunohistochemical techniques (PAP, IIT) showed amastigotes in groups or dispersed all over the tissues and/or fluorescence antigenic deposits, which allowed the observation of the effect of the infection in the sampled mice. PAP and IIT histochemical techniques also showed a higher sensitivity, detecting parasite and/or antigenic deposit in 95% and 86% respectively, as compared to 46% detected with H-E method. Tissue samples taken from re-inoculated mice did not show significant changes attributable to the effect of re-inoculation. In all samples, a lowered amount of amastigote forms in the heart and skeletal muscle, as well as a lesser degree of interstitial lymphomonocytic infiltrate, was observed as compared to those obtained in samples from the prime-infected animals. However, examined tissue samples following re-inoculation of primary T. cruzi-infected mice revealed positive results when IIT and PAP techniques were used, indicating the presence of antigenic deposits and/or parasite persistence (Fig.4 A-B-C).
PCR evaluation to confirm tissue parasite persistence
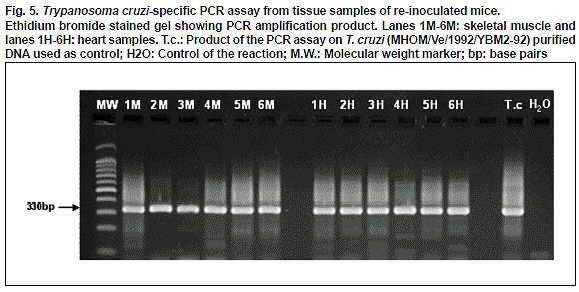
The molecular evaluation carried out using PCR assay supported the results presented above. The use of this molecular technique in heart and skeletal muscle samples corroborated the results obtained with the histochemical methods, indicating the parasite persistence in the selected tissues. The detection of specific T. cruzi DNA in muscle and heart samples of re-inoculated mice was evidenced by the amplification of a band of 330 bp as shown in Fig. 5.
In relation to mortality rates similar proportions were detected in the different groups. This included 51% during the course of T. cruzi-primary infection, 50% and 64% for those mice receiving first and second re-inoculations respectively, and 42% in the infected control group.
DISCUSSION
The present study evaluates the effect of T. cruzi-primary infection and two successive re-inoculations in a group of experimental mice.
The complete evaluation during the course of T. cruzi prime-infection showed a stage of the infection characterized by a significant presence of blood circulating trypomastigotes associated to the establishment of a humoral immune response with an early IgM transitorily elevated levels and a remarkable histopathological effect on the heart and other target organs in the infected mice. This pattern has been widely reported by many authors and recognized as the acute phase of T. cruzi-infection (Tarleton & Kuhn, 1983). Although the studied animals also showed to be susceptible to re-infections after succeeding T. cruzi
-prime infection, their exposure to successive re-inoculations with the same parasite did not reproduce the same profile detected during the acute phase. In this case, the parasitemia level in re-inoculated animals did not reach the same level detected in the primary infected mice, and a remarkable lesser amount of blood circulating parasites was recorded during this period of observation. A similar trend was also observed when T. cruzi-primary infected and re-inoculated mice were compared in terms of histopathological changes, including the distribution and amount of detected tissue amastigote forms and/or the degree of interstitial lymphomonocytic infiltrate. These findings have also been reported in rats exposed to successive re-inoculations (Revelli et al., 1990) which agree with the present study in that the low number of parasites and the extension of inflammatory lesions observed in T. cruzi re-inoculated animals may be attributable to a resistance response of the survivor mice. Similarly, when the level of detected IgG and IgM were compared, equivalent values were observed between the re-inoculated and infected control mice. The constant detection of IgG levels during the T. cruzi-primary infection following the parasitemia drop, and its maintenance after successive challenges, demonstrated a strong immunological response, which corroborate previous observations in the same murine model (Tarleton & Khun, 1983). The detected IgM pattern was very similar to the response reported for human infection (Añez et al., 2001) in which higher values of IgM during the acute phase of the infection, coinciding with the higher parasitemia level, was observed. It is worth mentioning that this observed Ig-pattern was constant during successive re-inoculations which suggest, on one hand, a time-stable response against T. cruzi and, on the other hand, that re-inoculations do not reproduce the initial acute phase of the American trypanosomiasis in its parasitological, serological and histopathological aspects. Analyzing the capability of a mammal host to resist a new T. cruzi-infection after succeeding a primary infection caused by the same parasite (i.e. clone, lineage, strain or isolate) as demonstrated here, it is possible to conclude that T. cruzi re-inoculations do not trigger a reactivation of infection similar to the typical acute clinical or immunological profiles.Taken all the present results together, it is possible to suggest that survivor primary-infection hosts should be able to prevent severe re-infection, establishing a protective stage against T. cruzi, making them resistant to a second infection. The above mentioned results find support in the work of previous authors who concluded that chronically T. cruzi-infected hosts are resistant to new re-infections irrespective of the species of the infected mammal and/or the genetic make up of the parasite (Brener, 1962; Nussenzweig et al., 1963; Lauria-Pires & Teixeira, 1997; Machado et al., 2001). Regarding the resistance to T. cruzi re-infection, this has been attributed to persisting infection, a phenomenon also known as premunition (Kagan & Norman, 1961). The parasite persistence was demonstrated in the present work in tissue samples from re-inoculated mice processed with immunohistochemical and molecular (PCR) techniques, that showed antigenic deposits in heart and skeletal muscle (in IIT staining), the parasite itself in the amastigote form (in PAP staining) and a portion of its genome (amplified by PCR) indicating that the detection of T. cruzi persistence in the murine model seems to be as frequent as that observed in the myocardium and other target tissues of chronic chagasic patients (Añez et al., 1999b; 2011). Thus, the premunition, described as a type of non-sterilizing immunity characterized by low amounts of persisting parasites in chronic infected individuals, should be a generalized phenomenon that explains the present results, which, at the same time, agrees with its occurrence in other parasitic diseases like Malaria (Perignon & Druilhe, 1994; Jones et al.
, 2000).The evidence presented here with the model
T. cruzi-mice finds support in similar results obtained with T. rangeli, another American trypanosome sharing same distribution and hosts with T. cruzi. In this case, experimental results obtained in different vertebrate models including mice, rats, marsupials and Proechymis, revealed that, once succeeded the primary infection by T. rangeli, these hosts became resistant to new infections probably due to a synergic action of both the cellular and humoral responses (Añez et al., 1985; Nieves & Añez, 1992). The coincidental occurrence of this similar phenomenon in both American species of Trypanosoma seems to suggest that it should be a frequent event in endemic areas. In fact, the report of this intriguing phenomenon, inextricably linked to the natural history of Chagas disease, was first reported nearly a century ago by Brumpt (1913) very shortly after the discovery of the disease, who demonstrated that survivor animals from an acute infection by T. cruzi developed a strong immunity to re-infection. The role of reinfection by this parasite in Chagas disease, thus, awaits further clarification.Finally, extrapolating the present experimental results to the human population, it is possible to foresee the epidemiological implication in areas where Chagas disease is endemic and where humans are constantly subjected to new infections by T. cruzi. The above seems to be reasonable considering the remarkable similarity between the infection in mice and the human chronic Chagas disease, previously reported (Basombrio et al., 1982). In this case, a primary T. cruzi
-infection, whatever its intensity, may serve as a natural protective factor to people suffering a severe re-infection by the same parasite. However, the possible emergence of reactivated or re-infected clinical cases in endemic areas due to constant exposition to new infections by different types, lineages or circulating clones of T. cruzi should not be ignored.CONFLICT OF INTERESTS
The authors declare no conflicts of interest.ACKNOWLEDGEMENTS
This work was supported by a grant from FONACIT (G-2005-000370, NA). GC is a Ph.D. student of the Biotechnology of Microorganisms Graduate Program, supported by FONACIT (grant 2006000457). Most information came from B.Sc. (Biol.) thesis of M.L.M. We are indebted to Dr. G. Fermín for his criticism and the careful reviewing of the manuscript.
REFERENCES
1. Añez N., Velandia J. & Rodríguez A. M. (1985). Estudios sobre
Trypanosoma rangeli TEJERA, 1920.VIII. Respuesta a las re-infecciones en dos mamíferos. Mem. Inst. Oswaldo Cruz. 80: 149-153. [ Links ]2. Añez N., Carrasco H., Parada H., Crisante G., Rojas A., González N.,
et al. (1999a). Acute Chagas disease in Western Venezuela. A clinical, seroparasitological and epidemiological study. Am. J. Trop. Med. Hyg. 60: 215-22. [ Links ]3. Añez N., Carrasco H., Parada H., Crisante G., Rojas A., Fuenmayor C.,
et al. (1999b). Myocardial parasite persistence in chronic chagasic patients. Am. J. Trop. Med. Hyg. 60: 726-732. [ Links ]4. Añez N., Crisante G., Rojas A., Carrasco H., Parada H., Yepez Y.,
et al. (2001). Detection and significance of inapparent infections in Chagas disease in western Venezuela. Am. J. Trop. Med. Hyg. 65: 227-232. [ Links ]5. Añez N., Crisante G., Maia da Silva F., Rojas A., Carrasco H., Umezawa E.S.,
et al. (2004). Predominance of lineage I among Trypanosoma cruzi isolates from Venezuela patients with different clinical profiles of acute Chagas disease. Trop. Med. Int. Health. 9: 1319-1326. [ Links ]6. Añez N., Crisante G., Caraballo F., Delgado W. & Parada H. (2011). Trypanosoma cruzi persistence at oral inflammatory foci in chronic chagasic patients. Acta Trop. 117:
207-211. [ Links ]7. Basombrio M. A., Besuschio S. & Cossio P. M. (1982). Side effects of immunization with live attenuated
Trypanosoma cruzi in mice and rabbits. Inf. Immun. 36: 342-350. [ Links ]8. Brener Z. (1962). Therapeutic activity and criterion of cure on mice experimentally infected with
Trypanosoma cruzi. Rev. Med. Trop. São Paulo. 4: 389-396. [ Links ]9. Brumpt E. (1913). Immunité partielle dans les infections à
Trypanosoma cruzi, transmission de ce trypanosome par Cimex rotundus. Rôle régulateur des la peau. Bull. Soc. Pathol. Exot. 6: 172-176. [ Links ]10. Bustamante J. M., Rivarola H. W., Fernandez A. R, Enders J. E., Fretes R., Palma J. A. & Paglini-Oliva P. A. (2002).
Trypanosoma cruzi re-infection in mice determines the severity of cardiac damage. Int. J. Parasitol. 32: 889-896. [ Links ]11. Bustamante J. M., Rivarola H. W., Fernandez A. R., Enders J. E., Fretes R., Palma J. A. & Paglini-Oliva P. A. (2003). Indeterminate Chagas´s disease:
Trypanosoma cruzi strain and re-infection are factors involved in the progression of cardiopathy. Clin. Sci. 104: 415-420. [ Links ]12. Dos Reis G. A. & Lopes M. F. (2000). A resposta imune à infecção pelo
Trypanosoma cruzi em modelos experimentais. In: Trypanosoma cruzi e doença de Chagas. Brener Z., Andrade Z. A. & Barral-Neto M. Eds. Guanabara Koogan. 2da Edição. Rio de Janeiro, Brasil. [ Links ]13. Jones T. R., Obaldia N., Gramzinski R. A. & Hoffman S. L. (2000). Repeated infection of Aoutus monkeys with
Plasmodium falciparum induces protection against subsequent challenge with homologous and heterologous strains of parasite. Am. J. Trop. Med. Hyg. 62: 675-680. [ Links ]14. Kagan I. G. & Norman L. (1961). Immunologic studies on
Trypanosoma cruzi. III. Duration of acquired immunity in mice initially infected with a North American strain of T. cruzi. J. Infect. Dis. 108: 213-217. [ Links ]15. Lauria-Pires L. & Teixeira A. R. (1997). Superinfections with genetically characterized Trypanosoma cruzi clones did not agravate morbidity and mortality in BALB/c mice. J. Parasitol. 83:
819-824. [ Links ]16. Machado E. M. M., Fernandes A. J., Murta S. M. E., Vitor R. W. A., Camilo Junior D. J., Pinheiro S.W.,
et al. (2001). A study of experimental re-infection by Trypanosoma cruzi in dogs. Am. J. Trop. Med. Hyg. 65: 958-965. [ Links ]17. Menezes H. (1969). Active immunization of dogs with a non virulent strain of
Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo. 11: 258-263. [ Links ]18. Menezes H. (1970). Essays on immunization of mice with ultraviolet radiated virulent and avirulent culture forms of
Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo. 12: 310-319. [ Links ]19. Menezes H. (1971). Aplicação de vacina viva avirulenta de
Trypanosoma cruzi em seres humanos. Rev. Inst. Med. Trop. São Paulo. 13: 144-154. [ Links ]20. Nieves E. & Añez N. (1992). Studies on
Trypanosoma rangeli TEJERA, 1920. XI. Protection trials in Wistar rats and Proechymis sp. Kasmera. 20: 53-72. [ Links ]21. Nussenzweig, V., Deane, L.M., Kloetzel, K. (1963). Differences in antigenic constitution of strains of
Trypanosoma cruzi. Exp. Parasitol. 14: 221-232. [ Links ]22. Perignon J. L. & Druilhe P. (1994). Immune mechanisms underlying the premunition against
Plasmodium falciparum. Mem. Inst. Oswaldo Cruz. 89: 51-53. [ Links ]23. Revelli S., Berra H., Valenti J., Moreno H., Bernasconi M., Poli H. & Moroni J. (1990). Efecto de la reinfección sobre la evolución de ratas infectadas con Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo. 32: 260-268. [ Links ]
24. Schijman, A.G., Bisio, M., Crisante, G., Añez, N., et al. (2011). International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Trop. Dis. 5: 1-13. [ Links ]
25. Silveira A. C. (2000). Profilaxia. In: Trypanosoma cruzi e doença de Chagas. Brener Z., Andrade Z. A. & Barral-Neto M. Eds. Guanabara Koogan. 2da Edição. Rio de Janeiro, Brasil. [ Links ]
26. Tarleton R. L. & Kuhn R. E. (1983). Changes in cell population and immunoglobulin-producing cells in the spleen of mice infected with Trypanosoma cruzi: correlation with parasite-specific antibody response. Cel. Immunol. 80: 392-404. [ Links ]
27. Zingales B., Andrade S. G., Briones M. R. S., Campbell D. A., Chiari E., Fernandes O., et al. (2009). A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 104: 1051-1054. [ Links ]












 uBio
uBio