Boletín de Malariología y Salud Ambiental
versão impressa ISSN 1690-4648
Bol Mal Salud Amb vol.54 no.2 Maracay dez. 2014
Biochemical and biological characterisation of lancehead (Bothrops venezuelensis Sandner 1952) snake venom from the Venezuelan Central Coastal range
Caracterización bioquímica y biológica del veneno de la serpiente "tigra mariposa" (Bothrops venezuelensis Sandner 1952) de la región central de la Cordillera de la Costa Venezolana
Elda E. Sánchez1, María E. Girón2, Nestor L. Uzcátegui2, Belsy Guerrero3, Max Saucedo1, Esteban Cuevas1 & Alexis Rodríguez-Acosta2*
1 National Natural Toxins Research Center, Texas A&M University-Kingsville, Kingsville, Texas, USA.
2 Laboratorio de Inmunoquímica y Ultraestructura Instituto Anatómico, Universidad Central de Venezuela, Caracas, Venezuela.
3 Laboratorio de Fisiopatología, Centro de Medicina Experimental, Instituto Venezolano de Investigaciones Científicas, Caracas 1020A, Venezuela.
*Autor de correspondencia: rodriguezacosta1946@yahoo.es
RESUMEN
Se aislaron fracciones del veneno de Bothrops venezuelensis que demuestran ser un espectro abundante de proteínas con actividades variadas (coagulante, hemorrágica, fibrinolítica, proteolítica y de función plaquetaria), para el análisis de sus propiedades físico-químicas y biológicas, el veneno fue fraccionado por cromatografía de exclusión molecular, corrido en una electroforesis en gel y realizada una batería de ensayos biológicos. La DL50 del veneno de B. venezuelensis fue 6,39 mg/kg de peso corporal, fue determinada inyectando intraperitonealmente en ratones, diluciones seriadas de veneno de B. venezuelensis. Se colectaron doce fracciones a partir del veneno de B. venezuelensis mediante cromatografía de exclusión molecular. Las fracciones 1-5 y 7-9 tenían actividad hemorrágica. Todas las fracciones, con la excepción de las fracciones 3 y 6, tenían actividad fibrinolítica. Ninguna de las fracciones tuvo actividad de gelatinasa significativa, y sólo fracciones 4-6 demostraron actividad en polvo azul de ocultamiento. Con la excepción de las fracciones 1 y 4 , todas hidrolizaron la cadena β de la insulina. Cada fracción del veneno, así como el veneno crudo mostraron actividad procoagulante, cuando se probó en un analizador Sonoclot. Las fracciones 1, 3 , 5 y 9 inhibieron la función plaquetaria. En este estudio se señalan actividades biológicas de un veneno poco estudiado (B. venezuelensis) y sus fracciones. Al detectar actividades hemorrágicas, fibrinolíticas, procoagulantes, proteolíticas y de inhibición de la función plaquetaria. Este estudio preliminar abre el camino para la identificación de moléculas específicas que podrían tener potencial terapéutico en hemostasia y cáncer, que vienen siendo estudiados en nuestro grupo.
Palabras clave: Bothrops venezuelensis, hemostasia, hemorragia, fibrinólisis, función plaquetaria, veneno.
SUMMARY
Venom fractions isolated from Bothrops venezuelensis were shown to contain a broad spectrum of proteins with varied activities. This study describes venom fractions with coagulant, haemorrhagic, fibrinolytic, proteolytic and antiplatelet activities, and analyses their physico-chemical properties and biological activities via molecular exclusion chromatography, gel electrophoresis and a bioassay battery. The LD50, determined by injecting intraperitoneally serial dilutions of B. venezuelensis venom into mice, was 6.39 mg/kg body weight. Twelve fractions were collected from B. venezuelensis venom using molecular exclusion chromatography. Of these, fractions 1-5 and 7-9 showed haemorrhagic activity, and all fractions except 3 and 6 showed fibrinolytic activity. However, none of the fractions had significant gelatinase activity, and only fractions 4-6 demonstrated activity on hide powder azure. With the exception of fractions 1 and 4, all fractions hydrolysed the insulin B-chain. In addition, all fractions as well as the crude venom showed strong procoagulant activity when tested using a Sonoclot Analyzer. Fractions 1, 3, 5 and 9 inhibited platelet function. In this study we have described the activities of the crude venom and its size-fractions from the scarcely studied B. venezuelensis. Haemorrhagic, fibrinolytic, procoagulant and proteolytic activities, and the inhibition of platelet function were detected. This preliminary study paves the way for the identification of specific molecules in B. venezuelensis venom that could have therapeutic potential for cancer and aberrant haemostasis treatment.
Key words: Bothrops venezuelensis, haemostasis, haemorrhages, fibrinolysis, platelet function, venom.
Recibido el 16/06/2014 Aceptado el 11/09/2014
Bothrops snake venoms are complex mixtures rich in proteins that include metalloproteinases, phospholipids A2, serine proteases and other proteases that act through different mechanisms. In bothropic envenomations, proteinases may interfere with the coagulation and fibrino(geno)lytic systems of victims, inducing systemic alterations of important relevance in the clinical picture (Assakura et al., 2003, Bello et al., 2006 , Salazar et al., 2007, Girón et al., 2013).
In Venezuela, Bothrops snakes cause 80% of human ophitoxemias with an important number of accidents among equine, goats and cattle resulting in severe economical loses (Rengifo and Rodríguez-Acosta, 2004). Bothropic accidents are multiple and severe, characterised by immediate local effects and different systemic manifestations where haemostasis disorders are predominant. These include coagulopathy through consumption as a result of thrombin-like molecules as well as activators of prothrombin and/or factor X. Another important feature in these patients is the activation of the fibrinolytic system with depletion of α2-antiplasmin and increase of products of degradation of fibrinogen and/or D-dimers. The main components responsible for these activities are metalloproteinases (Markland 1998).
There are two principal species of Bothrops snakes in the Venezuelan Central Coastal range. One is the well-studied Mapanare (an Amerindian name) (Bothrops colombiensis), and the other is the "tigra mariposa" (named due to their aggressive nature and butterfly like pattern on their scales) (Bothrops venezuelensis) (Rengifo and Rodríguez-Acosta 2004). However, the toxicity of B. venezuelensis snake venom is well-known, amid people living in their endemic area, by the severity of accidents and fatal bites among humans and cattle, but a comprehensive study of this snake and its venom has not been carried out. In the current work, toxic fractions from B. venezuelensis were identified and suggested to be an ample spectrum of proteins with diverse activities. This paper focuses on describing venom fractions with procoagulant, haemorrhagic, fibrinolytic, proteolytic and platelet function activities as well as analysing its physicochemical properties and biological activities. These analyses will provide a wealth of new information on these venom molecules to be characterised in the near future for the advancement of likely and supplementary efficient treatments as well as the enhancement of immunogens to produce better antivenoms.
MATERIALS AND METHODS
Venoms and snakes
Venoms were obtained from pools of fifteen B. venezuelensis snakes captured in the Venezuelan Central Coastal range at the Henri Pittier and Waraira Repano National Parks (Aragua state and Capital District, respectively). The snakes were kept in captivity in the Serpentarium of the Tropical Medicine Institute of the Universidad Central de Venezuela. Venoms were centrifuged to remove impurities, filtered and frozen at -70ºC until use.
Experimental animals
Rabbits
To test skin haemorrhagic activity New Zealand rabbits (Oryctolagus cuniculus) were used. Two kilogram weight rabbits were obtained from the Vivarium of Tropical Medicine Institute of the Universidad Central de Venezuela, Caracas and the National Natural Toxins Research Center of the Texas A & M University-Kingsville, Kingsville, Texas, USA.
Mice
Female mice (INH strain) weighing 18-20 g were purchased from the Instituto Nacional de Higiene "Rafael Rangel", Caracas, Venezuela. The colony of mice was kept in boxes in a room maintained at 23ºC on a 12/12-hr light/dark cycle.
Ethical statement
Professional staffs arranged all the experimental events concerning the use of live animals. Pertinent regulations as well as institutional guidelines, according to protocols approved by the National Natural Toxins Research Center, Texas A&M University-Kingsville, Texas, USA and the Institute of Anatomy of the Universidad Central de Venezuela following the norms obtained from the guidelines for the care and use of laboratory animals, published by the US National Institute of Health (NIH 1985).
Lethality assay
Five groups of eight mice for each venom were housed in cages and observed throughout the quarantine period and experiments. All venoms were pooled from the same species covering the same county. Venoms were dissolved in 0.85% saline at the highest concentration of venom that was used for injection. Two-fold serial dilutions using saline were made to obtain four additional concentrations. All solutions during the experiment were stored at 4ºC and warmed to 37ºC just before being injected into mice. The lethal toxicity was determined by injecting 0.2 mL of venom into the tail veins of 18-20 g female BALB/c mice. The injections were administered using a 1 mL syringe fitted with a 30-gauge, 0.5-in. needle. The saline control was used. The endpoint of lethality of the mice was determined after 48 h. The LD50 was calculated by the Spearman-Karber (1978) method.
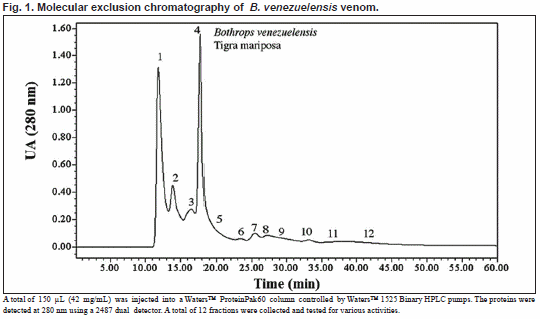
Molecular exclusion chromatography from B. venezuelensis venom
Four hundred micrograms of B. venezuelensis venom was separated by a 1-20 kDa molecular exclusion Waters ProteinPak60 (7.8 x 300 mm) column equilibrated with 0.02M sodium phosphate, pH 6.5 at a flow rate of 0.5 mL/min. The detection of proteins was spectrophotometrically carried out at 280 nm. A Waters High Performance Liquid Chromatography System (510 Pumps and a Tunable detector) was used.
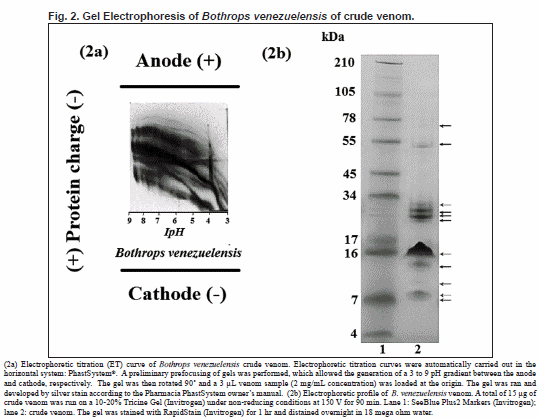
Electrophoretic titration curves (ET)
To determine the isoelectric points (pIs) for the proteins found in the venom of B. venezuelensis
, electrophoretic titration curves were employed. Samples were lyophilised and reconstituted in deionised water at 3 mg/mL. A pH gradient of 3-9 was established using IEF 3-9 PhastGels (GE Healthcare Life Sciences, Piscataway, NJ, USA).The gels were then rotated 90º and 3 μL samples were applied in the centre of the gel. The proteins were separated and silver stained as recommended the PhastSystem manuals.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
A total of 15 μg of crude venom was run on a 10-20% Tricine Gel (Invitrogen) under non-reducing conditions at 150 V for 90 min. Lane 1: SeeBlue Plus2 Markers (Invitrogen); lane 2: crude venom. The gel was stained with RapidStain (Invitrogen) for 1 hr and distained in 18 mega ohm water overnight.
Dialysis and protein concentration
Bothrops venezuelensis
venom and fractions obtained by chromatography were desalted using a Pharmacia G25 HiTrap column (5,000 Da molecular weight cutting), and concentrated by freeze-drying (6 Freezone Labconco, Kansas, MO, USA) at - 40ºC.Haemorrhagic analysis
To determine the haemorrhagic activities of B. venezuelensis crude venom and fractions, the modified Omori-Satoh et al. (1972) method was used. One hundred microlitres of crude venom or individual fractions were intracutaneously injected into the back of a New Zealand rabbit. After 15 h, the animal was sacrificed and depilated. Haemorrhagic activity was determined by the presence of haemorrhagic spots on the rabbits skin. Specific haemorrhagic activity was established by dividing the size of the haemorrhagic point (mm) by the quantity of injected protein (μg). Haemorrhagic activity was compared with minimum haemorrhagic dose (MHD: 2.5 μg) of Crotalus atrox crude venom, which is defined as the amount of venom that causes a 10-mm hemorrhagic spot. Saline solution was used as negative control.
Fibrinolytic analysis
To determine the fibrinolytic activity of B. venezuelensis venom fractions, the modified Bajwa et al. (1980) method was used. Three hundred microlitres of fibrinogen and 12 μL thrombin solutions were put in each well of a 24 well plate and softly agitated. The plate was kept at room temperature until content solidified and incubated at 37ºC for 3 h. Twenty microlitres of each fraction were added to each well and incubated at 37ºC for another 15 h. After incubation, 700 μL of 10% trichloroacetic acid was placed in each well to stop the reaction. The wells were emptied after 10 min and results observed.
Specific fibrinolytic activity was calculated by dividing the cleared fibrin area (mm2) by the amount of protein (μg) in each well.
Proteolytic analysis using hide powder azure
A modified hide powder azure method by Rinderknecht et al. (1968) was used to test the proteolytic activity of the venom. Eight milligrams of hide powder azure was diluted in 2 mL of 0.02 M Tris-HCl, pH 8.0, and 100 μL of venom fraction was added. Each sample was incubated at 37ºC for 1 h and agitated at intervals of 5 min. After incubation, each sample was centrifuged at 420 x g for 5 min. The supernatant was transferred to a vial and was measured at an absorbance of 595 nm. 0.02 M Tris-HCl was used as a negative control and C. atrox venom (1 mg/mL) was used as positive control. The absorbance units for each fraction were subtracted from the absorbance unit of the negative control. The specific activity was calculated by dividing the absorbance by the amount of used protein (mg).
Determination of the proteolytic activity of Bothrops venezuelensis venom fractions on B-chain of insulin using a capillary electrophoresis (CE)
To identify the proteolytic activity from each Bothrops venezuelensis venom fraction a P/ACE 5500 (Beckman, USA) capillary electrophoresis was used. Ten microlitres of the venom fractions (0.06 mg/mL) were incubated at room temperature for 1 h with 10 μL of B-chain of insulin (0.5 mg/mL) and 10 μL of 0. 1 M sodium borate, pH 8.3.Then the mixture was separated during 10 min at 20 kV, using 0.1 M sodium borate, pH 8.3, in a 75 μm I.D x 50 cm (800 x 100 aperture) capillary tube. The proteins were detected at 214 nm.
Gelatinase activity
A modified X-ray film method (Huang & Pérez, 1980) was employed to assay the gelatinase activity of B. venezuelensis crude venom and fractions. An X-ray film containing a gelatine coating (Kodak X-OMAT) was washed with distilled water
and incubated at 37ºC for 45 min. After incubation, the film was completely dried and 20 μL of sequential diluted crude venom or fractions (starting at 50 μg protein) were placed on the film. The film was incubated for 2 h at 37ºC in a humid incubator. After incubation, the film was washed with distilled water and observed for cleared areas indicating hydrolysis. Serial dilutions were performed to determine the minimum amount of venom required to cause a clear spot on the film. The titre was defined as the reciprocal of the highest dilution that caused a clear spot on the film. The specific gelatinase activity was calculated by dividing the titre by the amount of protein (μg) applied on the film.Blood sample collection
Blood was collected using a gravity flowing system, which generated no trauma to platelets. Eighteen millilitres of human blood (obtained with proper donor consent) were collected in a 50 mL test tube containing 1.8 mL of 1% sodium citrate. After blood collection, the tube was lightly inverted twice to ensure the total blood citration. The blood was aliquoted in 2 mL samples prior to its use.
Plasma collection
Fresh human plasma was obtained from our laboratory blood samples donors, without alterations in haemostasis, which was anticoagulated with 3.8% sodium citrate in 1:9 ratio. Plasma was obtained by centrifugation at 2000 g for 15 minutes at 4ºC.
Procoagulant activity
To test the B. venezuelensis crude venom coagulant activity, bovine fibrinogen or fresh frozen plasma was used as substrate to evaluate thrombin-like or procoagulant activity (Austen & Rhymes, 1975). Briefly, 100 μL of citrated plasma or 0.3% fibrinogen solution was used in 0.05 M Tris -HCl pH 7.4 buffer (coagulation) by incubating for 3 min at 37ºC, then adding 100 μL of coagulation buffer and 100 μL of thrombin solution (0.5 to 15 IU/mL) or 100 μL of crude venom or fraction (100 μg /mL); the mixture was hand agitated at 37ºC and the coagulation time was recorded. Thrombin solution adjusted to 2.5 IU/mL prepared with coagulation buffer in which the clotting time is recorded between 18 and 22s is used as a control.
The thrombin-like activity present in the crude venom was reported in IU/mL when extrapolating results in a calibration curve generated with a thrombin control.
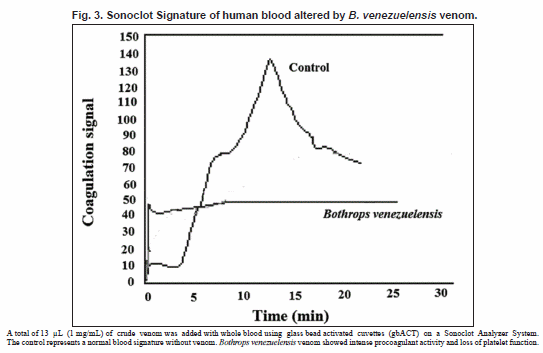
Coagulant and platelet function activity of venom and venom fractions via Sonoclot Analysis
Activation time, rate of coagulation and platelet function were analysed using a glass bead activated kit (gbAC, Sienco®, USA) on a Sonoclot® and a Platelet Function Analyser (Sienco®, Inc.
Wheat Ridge, CO, U.S.A). A total of 300 μL of 37º human citrated blood was added to a glass bead activated cuvette containing 13 μ of 0.25 M CaCl2 and 13 μ of venom (1mg/mL) or venom fraction (varying concentrations as collected from molecular exclusion). The data was evaluated using a "Signature Viewer provided by Sienco® on an IMac computer.RESULTS
Lethality assay
The LD50 calculated from the B. venezuelensis snake venom was 6.39 mg/kg.
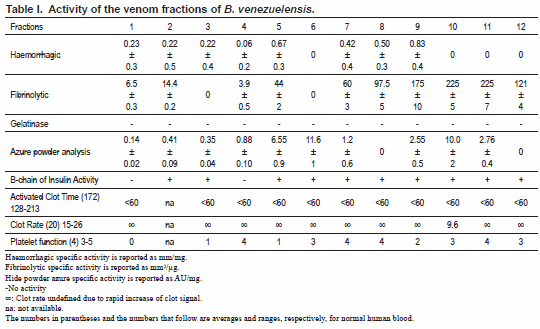
Molecular exclusion chromatography from B. venezuelensis venom Twelve fractions were collected from B. venezuelensis venom (Fig. 1). Table 1 shows all fractions with their corresponding activities.
Electrophoretic titration curves (ET)
A banding pattern (Fig. 2a) gave us broad information on variations in the surface charge and pI of the venom proteins at different pHs. The titration curved showed that B. venezuelensis venom was a mixture of complex molecules many of which were acidic proteins. Although the ET is used primarily to determine the optimal conditions for the separation of molecules by ion exchange chromatography, it can also be used to indicate purity and venom complexity.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
A total of 11 bands were observed ranging between 70 and 7 kDa (Fig. 2b).
Haemorrhagic analysis
Haemorrhagic activity was only detected in venom fractions 1-5, and 7-9. Fractions, 5, and 7-9 had the highest haemorrhagic specific activities (Table I).
Fibrinolytic activity in fibrin plates
Fibrinolytic activity of crude B. venezuelensis venom on fibrin plates was 11.0
(mm²/μg). Fibrinolytic activity was eluted in venom fractions 1, 2, 4, 5, 7-12 (Table I). The highest specific fibrinolytic activity was observed in fractions 8-12.
Gelatinase activity
The crude venom along with the chromatographic fractions was void of gelatinase activity (Table I).
Proteolytic analysis using hide powder azure
Crude B. venezuelensis venom had hide powder azure activity of 5.98 UA/mg proteins. All fractions, with the exception of 8 and 12, contained activity; however, fractions 5, 6 and 10 had the highest specific activity (Table I).
Determination of the proteolytic activity of B. venezuelensis venom fractions using a capillary electrophoresis (CE)
With the exception of fractions 1 and 4, all B. venezuelensis venom fractions had proteolytic activity by cleaving oxidized B-chain of insulin (Table I).
Procoagulant activity
The procoagulant activity was evaluated on citrated human plasma or purified human fibrinogen. B. venezuelensis activity on plasma was 58.0 IU Thr/μg. When using purified human fibrinogen as substrate, B. venezuelensis venom showed 40.0 IU Thr/μg.
Coagulant and platelet function activity of venom and venom fractions via Sonoclot Analysis
All venom fractions had procoagulant activity when tested by the Sonoclot Analyzer. All fractions, including the crude venom, activated coagulation before 60 seconds (Table I). Normal activated clot time ranges from 128-213 s. The clot rate was undefined for all samples, which is a result of the immediate rise of the clot signal signifying strong coagulant activity (Fig. 3).
Platelet function (PF) is a measurement of the quality of clot retraction that causes a peak or peaks on the Sonoclot Signature. Platelet function is assigned a number from 0-5, where 0 signifies no PF and 5 indicates very strong PF. Normal PF varies anywhere from 3-4. Crude venom had a PF of 0. A PF of 0 was also observed for fraction 1. Fractions 3, 5, and 9 had PFs of less than the normal range (Table I), and all other fractions displayed normal PFs.
DISCUSSION
Many of the early efforts of venom research have been directed toward the isolation and description of any venom protein that was found in abundance or those containing the most toxic activities. With the advent of more sophisticated techniques of purification, there have been studies of the new and interesting components of protein found in smaller quantities. Snake venom encloses many components with different pharmacological and biological activities, especially in haemostasis and anti-tumour therapy, and has progressively become a research motivation. A quantity of effective components like disintegrins (in particular anticoagulants), antitumor molecules and pain-relieving factors has been recently described from snake venom (Xu et al., 2005; Seoane et al., 2007, Zhang & Rui, 2007; Calvete et al., 2009; Sánchez et al., 2009). The synergic action of the venom proteins can enhance their activities or contribute to the spread of toxins (Calvete et al., 2009), and this type of synergy plays an important role on the toxicity of venoms.
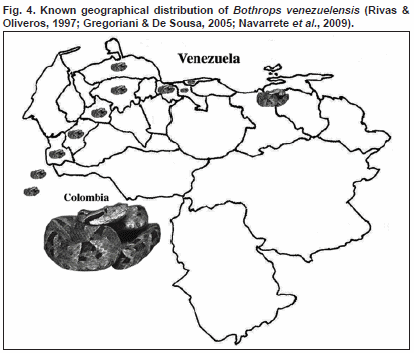
Bothrops venezuelensis, belonging to the Bothrops complex in South America, is one of the important snakes in the mountain areas of northern, eastern and western Venezuela as well as eastern Colombia (Fig. 4). Bothrops venezuelensis venom, as other bothropic venoms, contains numerous proteins that provoke, in envenomed victims, haemostatic alterations involving platelets, the coagulation and fibrinolysis systems as well as proteolytic and necrosis causing haemorrhagic syndrome and tissue death, respectively (Rengifo & Rodríguez-Acosta, 2004; Gutiérrez et al., 2009). The study of this venom has been neglected and scarcely investigated.
In the current paper, B. venezuelensis venom was fractionated by molecular exclusion Waters Protein-Pak60 chromatography. Procoagulant, fibrinolytic, haemorrhagic and platelet function activities were detected in the fractions of this venom. The molecular weights were determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and liquid chromatography.
Haemorrhage is a common manifestation in human and animal victims following a bite by B. venezuelensis (Rengifo & Rodríguez-Acosta, 2004). Of the 12 fractions collected by size exclusion chromatography, strong haemorrhagic specific activity was detected in fraction 8, which represented 66.7% of the venom components. Many snake venom components such as metallo- and serine-proteinases (Markland, 1998) produce local haemorrhage by direct or indirect actions on blood vessel membranes. On the other hand, these proteinases with important proteolytic functions represent significant tools for investigating the mechanism involved in blood vessel injury. The venom of B. venezuelensis presented a minimal haemorrhagic dose (MHD) of 2.5 μg. This potent dose could be associated with high molecular weight metalloproteinases such as Bothropasin, a type P-III metalloproteinase with potent haemorrhagic and necrotic activities, found in the venom of B. jararaca (Assakura et al., 2003). The haemorrhagic activity of B. venezuelensis is similar to the MHD of the Western diamondback rattlesnake venom.
The specific toxins that disrupt blood coagulation activity may inhibit platelet aggregation or may digest fibrin and/or fibrinogen as well as other coagulation factors in blood. In the current work, B. venezuelensis venom had 12 fractions that eluted by molecular exclusion chromatography. Fractions 3 and 6 displaying fibrinolytic activity. Fractions 5, and 7-12 contained the highest specific activity. It is important to emphasize that fibrinolytic venom components, after purification and characterization could be used therapeutically against various coagulation disorders.
Fibrinolytic enzymes that cause lyses of blood clots by direct fibrin degradation or plasminogen activators have been reported in the Viperidae family (Bello et al., 2006; Salazar et al., 2007; Girón et al., 2013). Nevertheless, no report has been described on the fibrinolytic activity in the venom of B. venezuelensis.
In addition, B. venezuelensis venom displayed proteolytic activity on substrates such as hide powder azure and B-chain of insulin, but had poor collagenases activity since no activity was observed on the X-ray film despite knowing that bothropic venoms have collagenase activity as demonstrated by zymograms (Giron et al., 2013).
Coagulation disorders are more prominent in those bitten by Bothrops. In this work, it was observed that B. venezuelensis venom is comprised of differen venom components, which can either stimulate or inhibit the blood coagulation pathway. All fractions activated coagulation before 60 seconds and the rate of coagulation was not registered due the rapid speed of clot formation signifying strong procoagulant activity. Several published papers report that the coagulation activities of snake venom proteins are attributed to a number of venom molecules, such as inhibitors of blood coagulation factors IX and X, activation of protein C, inhibitors of thrombin, α and β-fibrinogenases (rarely γ- fibrinogenases), serine proteinases and L-amino acid oxidases (Larréché et al., 2008; Rodríguez-Acosta et al., 2010; Girón et al., 2013) all degrade fibrinogen, and phospholipases damage phospholipids responsible for the formation of complexes vital to the activation of the coagulation cascade (Nahas et al.,1979; Kini 2006,).
Considering the molecular weights as evidenced by electrophoresis or by gel filtration chromatography, the proteins present in B. venezuelensis may represent components with enzymatic activity similar to those reported in Bothrops venoms described to date, which have been evidenced as metalloproteinases with haemorrhagic activity (64-40 kDa), serine proteinases (40-20 kDa) and phospholipase A2 (16-13 kDa) (Moura da Silva et al., 1990).
Bothrops venezuelensis venom showed 11 protein bands between 78-45 kDa (2), between 34-17 kDa (4), and between 16 and 7 kDa (5). Similar results are shown in inter specific studies of variability of Brazilian Bothrops snake venoms in which the study included Bothrops alternatus, B. atrox, B. bilineatus, B. brazili, B. castelnaudi, B. cotiara, B. erythromelas, B. fonsecai, B. hyoprorus, B. insularis, B. itapetiningae, B. jararaca, B. jararacussu, B. leucurus, B. marajoensis, B. moojeni, B. neuwiedi, B. pirajai and B. pradoi. The venom of these 19 species differed in composition, number and intensity of the protein bands via electrophoresis. However most of the venoms contained components, with molecular masses between 64 and 25 kDa, and about 14 kDa (Queiroz et al., 2008).
Furtado (2005), comparing B. jararaca and B. alcatraz venoms, exhibited similar electrophoretic profiles. However, B. alcatraz displayed three protein bands of molecular masses of 97, 80 and 38 kDa, which were not present in the venom of B. jararaca.
These proteins bands could be associated with the stronger coagulant and proteolytic activities reported in the venom of B. alcatraz.
The clotting activity analysis from B. venezuelensis venom using plasma as substrate was 58 IUThr/μg and was higher for that of B. colombiensis in 2 regions of Venezuela (Girón et al., 2008). The results of this clotting activity suggest the possible presence of thrombin-like proteins and factor X activators as has been reported by other authors (Nahas et al., 1979, Maruyama et al., 1992; Sánchez et al., 2010), and/or prothrombins as reported for B. asper (Loria et al., 2003); B. erythromelas (Silva et al., 2003); B. insularis (Modesto et al., 2005); B. cotiara (Senis et al., 2006); and B. jararaca (Berger et al., 2008).
It was in our best interest to initially identify those fractions containing strong inhibition of platelet function and having very little or no proteolytic activity. We are aware that the initial fractions consist of heterogeneous mixtures of proteins that could contain disintegrins (inhibitors of platelet function) as well as proteolytic enzymes (e.g. metalloproteinases, serine proteases, phospholipase A2s, etc.). The fractions fitting the criteria of strong platelet function inhibitors and low to no proteolytic activity were fractions 1 and 3. However, it is the intention after this preliminary research to allow us to identify many important activities which can be used as a foundation for later investigations on these venom molecules that have a promising future in biomedical research. The application of Bothrops venom in haemostasis and cancer therapy has caused a research frenzy, since many venom constituents have a wide range of uses in scientific research and in human and veterinary medicine.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
ACKNOWLEDGEMENTS
Funding for the research was provided by grants from the Science and Technology Fund (FONACIT) programs (PG-2005000400 grant), Instituto Venezolano de Investigaciones Científicas,
Caracas, Venezuela. NCRR/BMRG, Viper Resource Grant #s 8P40OD01960-10 and 3P40OD01096-10S1 (NNTRC, Texas A&M University-Kingsville), and the Robert A. Welch Foundation Department Grant # AC-0006 (TAMUK-Department of Chemistry).
REFERENCES
1. Assakura M. T., Silva C. A., Mentele R., Camargo A. C. & Serrano S. M. (2003). Molecular cloning and expression of structural domains of bothropasin, a P-III metalloproteinase from the venom of Bothrops jararaca. Toxicon. 41: 217-27. [ Links ]
2. Austen D. & Rhymes I. A. (1975). In: Mead, O. (Ed.), Laboratory Manual of Blood Coagulation. Blackwell Scientific Publications, Oxford, England.
3. Bajwa S. S., Markland F. S. & Russell F. E. (1980). Fibrinolytic enzyme(s) in western diamondback rattlesnake (Crotalus atrox) venom. Toxicon. 18: 285-290. [ Links ]
4. Bello C. A., Hermogenes A. L., Magalhaes A., Veiga S. S., Gremski L. H., Richardson M., et al. (2006). Isolation and biochemical characterization of a fibrinolytic proteinase from Bothrops leucurus (white-tailed jararaca) snake venom. Biochimie. 88: 189-200. [ Links ]
5. Berger M., Pinto A.F. & Guimarães J.A. (2008). Purification and functional characterization of bothrojaractivase, a prothrombin-activating metalloproteinase isolated from Bothrops jararaca snake venom. Toxicon. 51: 488-501. [ Links ]
6. Calvete J. J., Borges A., Segura A., Flores-Díaz M., Alape-Girón A., Gutiérrez J. M., et al. (2009). Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snakebite management. J. Proteomics. 72: 227-240. [ Links ]
7. Furtado M. F. (2005). Biological and immunological properties of the venom of Bothrops alcatraz, an endemic species of pitviper from Brazil. Comp. Biochem. Physiol C. Toxicol. Pharmacol. 141: 117-123. [ Links ]
8. Girón M. E., Salazar A. M., Aguilar I., Pérez J. C., Sánchez E. E., Arocha-Piñango C. L., et al. (2008). Hemorrhagic, coagulant and fibrino(geno)lytic activities of crude venom and fractions from mapanare (Bothrops colombiensis) snakes. Comp. Biochem. Physiol C. Toxicol. Pharmacol. 147: 113-121. [ Links ]
9. Girón M. E., Guerrero B., Salazar A. M., Sánchez E. E., Alvarez M. & Rodríguez-Acosta A. (2013). Functional characterization of fibrinolytic metalloproteinases (colombienases) isolated from Bothrops colombiensis venom. Toxicon. 74: 116-26. [ Links ]
10. Gregoriani T. & De Sousa L. (2005). Sima Talpa Bothrops, serranía del Turimiquire, estado Anzoátegui, Venezuela. Saber. 17: 84-87. [ Links ]
11. Gutiérrez J. M., Escalante T. & Rucavado A. (2009). Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon. 54: 976-987. [ Links ]
12. Huang S. Y. & Perez J. C. (1980). Comparative study on hemorrhagic and proteolytic activities of snake venoms. Toxicon. 18: 421-426. [ Links ]
13. Kini R. M. (2006). Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem. J. 397: 377-387. [ Links ]
14. Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680-685. [ Links ]
15. Larréché S., Mion G. & Goyffon M. (2008). Haemostasis disorders caused by snake venoms. Ann. Fr. Anesth. Reanim. 27: 302-309. [ Links ]
16. Loria G. D., Rucavado A., Kamiguti A. S., Theakston R. D, Fox J. W., Alape A., et al. (2003). Characterization of 'basparin A,' a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Arch. Biochem. Biophys. 418: 13-24. [ Links ]
17. Markland Jr. F. S. (1998). Snake venom fibrinogenolytic and fibrinolytic enzymes: an updated inventory. Thromb. Haemost. 79: 668-674. [ Links ]
18. Maruyama M., Kamiguti A. S., Tomy S. C., Antonio L. C., Sugiki M. & Mihara H. (1992). Prothrombin and factor X activating properties of Bothrops erythromelas venom. Ann. Trop. Med. Parasitol. 86: 549-556. [ Links ]
19. Modesto J. C., Junqueira-de-Azevedo I. L., Neves-Ferreira A. G., Fritzen M., Oliva M. L., Ho P. L., et al. (2005). Insularinase A, a prothrombin activator from Bothrops insularis venom, is a metalloprotease derived from a gene encoding protease and disintegrin domains. Biol. Chem. 386: 589-600. [ Links ]
20. Morita T. (2005). Structures and functions of snake venom CLPs (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon. 45: 1099-1114. [ Links ]
21. Moura-da-Silva A. M., Cardoso D. F & Tanizaki M. M. (1990). Differences in distribution of myotoxic proteins in venoms from different Bothrops species. Toxicon. 28: 1293-1301. [ Links ]
22. Nahas L., Kamiguti A. S. & Barros M. A. (1979). Thrombin-like and factor X-activator components of Bothrops snake venoms. Thromb. Haemost. 41: 314-328. [ Links ]
23. Navarrete L. F., López-Johnston J. C. & Blanco-Dávila A. (2009). Guía de las serpientes de Venezuela. Biología, venenos, conservación y listado de especies. Gráficas ACEA, Caracas, Venezuela, 103 pp.
24. NIH, Principles of Laboratory Animal Care (1985). National Institute of Health of United States, Maryland, p. 1-86.
25. Omori-Satoh T., Sadahiro S., Ohsaka A. & Murata R. (1972). Purification and characterization of an antihemorrhagic factor in the serum of Trimeresurus flavoviridis, a crotalid. Biochim. Biophys. Acta. 285: 414-426. [ Links ]
26. Queiroz G. P., Pessoa L. A., Portaro F. C., Furtado Mde F. & Tambourgi D. V. (2008). Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon. 52: 842-851. [ Links ]
27. Rengifo C. & Rodríguez-Acosta A. (2004). Serpientes, venenos y su tratamiento en Venezuela. Fondo de Publicaciones de la Facultad de Medicina de la Universidad Central de Venezuela, Caracas, p.1-80.
28. Rinderknecht H., Geokas M. C., Silverman P. & Haverback B. J. (1968). A new ultrasensitive method for the determination of proteolytic activity. Clin. Chim. Acta. 21: 197-203. [ Links ]
29. Rivas G. & Oliveros O. (1997). Herpetofauna del Estado Sucre, Venezuela: Lista preliminar de reptiles. Soc. Cien. Nat. La Salle. 57: 67-80. [ Links ]
30. Rodríguez-Acosta A., Márquez A., Salazar A. M., Girón M. E., Carvajal Z., Ibarra C., et al. (2010). Hemostatic properties of Venezuelan Bothrops snake venoms with special reference to Bothrops isabelae venom. Toxicon. 56: 926-935. [ Links ]
31. Salazar A. M., Rodríguez-Acosta A., Girón M. E., Aguilar I. & Guerrero B. (2007). A comparative analysis of the clotting and fibrinolytic activities of the mapanare (Bothrops atrox) snake venom from different geographical areas in Venezuela. Thromb. Res. 120: 95-104. [ Links ]
32. Sánchez E. E., Rodríguez-Acosta A., Palomar R., Lucena S. E., Bashir S., Soto J. G., et al. (2009). Colombistatin: a disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Arch. Toxicol. 83: 271-279. [ Links ]
33. Sánchez E. E., Rodríguez-Acosta A., Cantu E. & Guerrero B. (2010). Antivenoms and Coagulation. In: Kini R. M., Clemetson K. J., Markland F. S.,
34. McLane M. A., Morita. Toxins and Hemostasis from Bench to Bedside. Editorial Springer Dordrecht Heidelberg London New York.
35. Senis Y. A., Kim P. Y., Fuller G. L. J., Garcia A., Prabhakar S., Wilkinson M. C., et al. (2006). Isolation and characterization of cotiaractivase, a novel low molecular weight prothrombin activator from the venom of Bothrops cotiara. Biochim. Biophys. Acta. 1764: 863-871. [ Links ]
36. Seoane A. I., Tran V. L., Sánchez E. E., White S. A., Choi J. L., Gaytán B., et al. (2007). The mojastin mutant Moj-DM induces apoptosis of the human melanoma SK-Mel-28, but not the mutant Moj-NN nor the non-mutated recombinant Moj-WN. Gene. 389: 66-72. [ Links ]
37. Silva M. B., Schattner M., Ramos C. R., Junqueira-de-Azevedo I. L., Guarnieri M. C., Lazzari M. A., et al. (2003). A prothrombin activator from Bothrops erythromelas (jararaca-da-seca) snake venom: characterization and molecular cloning. Biochem. J. 369: 129-139. [ Links ]
38. Spearman-Karber R. (1978). Alternative methods of analysis for quantal responses. In: Finney, D. (Ed.), Statistical Method in Biological Assay. Charles Griffin, London.
39. Xu W., Li Z. Q., Wu S. Y. & Wu S. G. (2005). Structural and functional characterization of snake venom disintegrins. Chin. Pharmacol. Bull. (Chin). 21: 17-22. [ Links ]
40. Zhang Y. & Rui J. (2007). Research progress of snake venom disintegrin in treatment of tumor. Chin. J. Clin. Pharmacol. Ther. (Chin). 12: 984-988. [ Links ]












 uBio
uBio